Abstract
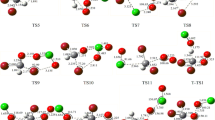
Recent studies suggest that the destruction of methane by Cl• in the marine boundary layer could be accounted for as another major sink besides the methane destruction by OH•. High level ab initio molecular orbital calculations have been carried out to study the CH4+Cl• reaction, the carbon Kinetic Isotope Effect (KIE) is calculated using Conventional Transition-State Theory (CTST) plus Wigner and Eckart semiclassical tunneling corrections. The calculated KIE is around 1.026 at 300 K and has a small temperature variation. This is by far the largest KIE among different processes involving atmospheric methane destruction (e.g., OH•, soil). A calculated mass balance of atmospheric methane including the KIE for the CH4+Cl• reaction is found to favor those methane budgets with enhanced biological methane sources, which have relatively lighter carbon isotope composition.
Similar content being viewed by others
References
Cantrell, C. A., Shetter, R. E., McDaniel, A. H., Calvert, J. G., Davidson, J. A., Tyler, S. C., Greenberg, J. P., and Lowe, D. C., 1990, Carbon kinetic isotope effect in the oxidation of methane by the hydroxyl radical, J. Geophys. Res. 95, 22455–22462.
Cicerone, R. J. and Oremland, R. S., 1988, Biogeochemical aspects of atmospheric methane, Global Biogeochemical Cycles 2, 299–327.
Coleman, D. D., Risatti, J. B., and Shoell, M., 1981, Fractionation of carbon and hydrogen isotopes by methane-oxidizing bacteria, Geochim. Cosmochim. Acta 45, 1033–1037.
Craig, H., Chou, C. C., Welhan, J. A., Stevens, C. M., and Engelkemeir, A., 1988, The isotopic composition of methane in polar ice cores, Science 242, 1535–1539.
Crutzen, P. J., 1991, Methane's sinks and sources, Nature 350, 380–381.
DeMore, W. B., Sanders, S. P., Golden, D. M., Hampson, R. F., Kurylo, M. J., Howard, C. J., Ravishankara, A. R., Kolb, C. E., and Molina, M. J., 1992, Chemical kinetics and photochemical data for use in stratospheric modeling, JPL Publ. 92-10, NASA.
Frisch, M. J., Head-Gordon, M., Foresman, J. B., Trucks, G. W., Raghavachari, K., Schlegel, H. B., Robb, M. A., Binkley, J. S., Gonzalez, C., Defrees, D. J., Fox, D. J., Whiteside, R. A., Seeger, R., Melius, C. F., Baker, J., Kahn, L. R., Stewart, J. J. P., Fluder, E. M., Topiol, S., and Pople, J. A., 1990, Gausian 90, Gaussian, Inc., Pittsburg, PA 15213.
Frisch, M. J., Pople, J. A., and Binkley, J. S., 1984, Self-consistent molecular orbital methods 25, Supplementary functions for Gaussian basis sets, J. Chem. Phys. 80, 3265–3269.
Frisch, M. J., Trucks, G. W., Head-Gordon, M., Gill, P. M. W., Wong, M. W., Foresman, J. B., Johnson, B. G., Schlegel, H. B., Robb, M. A., Replogle, E. S., Gomperts, R., Andres, J. L., Raghavachari, K., Binkley, J. S., Gonzalez, C., Martin, R. L., Fox, D. J., Defrees, D. J., Baker, J., Stewart, J. J. P., and Peopo, J. A., 1992, Gaussian 92, Gaussian, Inc., Pittsburgh, PA 15213.
Fung, I., John, J., Lerner, J., Matthews, E., Prather, M., Steele, L. P., and Fraser, P. J., 1991, Three-dimensional model synthesis of the global methane, J. Geophys. Res. 96 (D7), 13033–13065.
Gonzalez, C., McDouall, J. J. W., and Schlegel, H. B. 1990, Ab initio study of the reaction between methane and OH, H, and 3O, J. Phys. Chem. 94, 9467–7471.
Hehre, W. J., Radom, L., Schleyer, P. R., and Pople, J. A., 1986, Ab initio Molecular Orbital Theory, Wiley, New York.
Houghton, J. T., Jenkins, G. J., and Ephraums, J. J. (eds.), 1990, Climate Change: The IPCC Scientific Assessment, Cambridge Press, Cambridge, 337 pp.
JANAF Thermochemical Tables, 3rd edn., ed. by Chase, M. W. Jr., Davies, C. A., Downey, J. R. Jr., Frurip, D. J., and McDonald, R. A. (Natl. Stand. Ref. Data Ser. Natl. Bur. Stand., 1985), Vol. 14.
Johnston, H. S., 1966, Gas Phase Reaction Rate Theory, Ronald Press, New York.
King, S. L., Quay, P. D., and Lansdown, J. M., 1989, The 13C/12C kinetic isotope effect for soil oxidation of methane at ambient atmospheric concentrations, J. Geophys. Res. 94 (D15), 18273–18277.
Kreevoy, M. M. and Truhlar, D. G., 1986, Transition state theory, in investigation of rates and mechanisms of reactions, in C. F. Bernasconi (ed.), Techniques of Chemistry, Vol. VI, Wiley-Interscience, New York.
Lasaga, A. C. and Gibbs, G. V., 1991, Ab initio studies of the kinetic isotope effect of the CH4+OH• atmospheric reaction, Geophys. Res. Lett. 18, 1217–1220.
Melissas, V. S. and Truhlar, D. G., 1993a, Interpolated variational transition state theory and tunneling calculations of the rate constant of the reaction OH+CH4 at 223–2400 K, J. Chem. Phys. 99 (2), 1013–1027.
Melissas, V. S. and Truhlar, D. G., 1993b, Deuterium and carbon-13 kinetic isotope effects for the reaction of OH with CH4, J. Chem. Phys. 99 (5), 3542–3552.
Martin, J. M. L., Francois, J. P., and Gijbels, R., 1989, Combined bond-polarization basis sets for accurate determination of dissociation energies, II. Basis set superposition error as a function of the parent basis set, J. Comput. Chem. 10, 875–886.
Moore, J. W. and Pearson, R. G., 1981, Kinetics and Mechanisms, Wiley, New York, 455 pp.
Pszenny, A. A. P., Keene, W. C., Jacob, D. J., Fan, S., Maben, J. R., Zetwo, M. P., Springer-Young, M., and Galloway, J. N., 1993, Evidence of inorganic chlorine gases other than hydrogen chloride in marien surface air, Geophys. Res. Lett. 20 (8), 699–702.
Quay, P. D., King, S. L., Lansdown, J. M., Wilbur, D. O., 1988, Isotopic composition of methane released from wetlands: Implications for the increase in atmospheric methane, Global Biogeochemical Cycles 2, 385–397.
Quay, P. D., King, S. L., Stutsman, J., Wilbur, D. O., Steele, L. P., Fung, I., Gammon, R. H., Brown, T. A., Farwell, G. W., Grootes, P. M., and Schmidt, F. H., 1991, Carbon isotopic composition of atmospheric methane: fossil and biomass burning source strengths, Global Biogeochemical Cycles 5 (1), 25–47.
Rust, F. E., 1981, Ruminant methane δ(13C/12C) values: relation to atmospheric methane, Science 211, 1044–1046.
Sauer, J., 1989, Molecular models in ab-initio studies of solids and surfaces: from ionic crystals and semiconductors to catalysis, Chem. Rev. 89, 199–255.
Schlegel, H. B., 1986, Potential energy curves using unrestricted Møller-Plesset perturbation theory with spin annihilation, J. Chem. Phys. 84, 4530–4534.
Stevens, C. M. and Rust, F. E., 1982, The carbon isotopic composition of atmospheric methane, J. Geophys. Res. 87, 4879–4882.
Stevens, C. and Engelkemeir, A., 1988, Stable carbon isotopic composition of methane from some natural and anthropogenic sources, J. Geophys. Res. 93 (D1), 725–733.
Truong, N. T., and Truhlar, D. G., 1990, Ab initio transition state theory calculations of the reaction rate OH+CH4→H2O+CH3, J. Chem. Phys. 93, 1761–1769.
Tully, F. P., Ravishankara, A. R., Thompson, R. L., Nicovich, J. M., Shah, R. C., Kreutter, N. M. and Wine, P. H., 1981, Kinetics of the reactions between hydroxyl radical with benzene and toluene, J. Phys. Chem. 85, 2262–2269.
Tyler, S. C., 1986, Stable carbon isotope ratios in atmospheric methane and some of its sources. J. Geophys. Res. 91 (D12), 13232–13238.
Tyler, S. C., 1987, 13C/12C ratio in methane from the flooded Amazon forest, J. Geophys. Res. 92 (D1), 1044–1048.
Tyler, S. C., 1992, Kinetic isotope effects and their use in studying atmospheric trace species; case study, CH4+OH, in: Isotope Effects in Gas-Phase Chemistry, American Chemical Society, pp. 390–408.
Van Hook, W. A., 1970, Kientic isotope effect: Introduction and discussion of the theory, in C. J. Collins and N. S. Bowman (eds.), Isotope Effect in Chemical Reactions, Van Nostrand Reinhold Co., New York.
Wahlen, M., Tanaka, N., Henry, R., Deck, B., Zeglen, J., Vogel, J. S., Southern, J., Shemesh, A., Fairbanks, R., and Broecker, W., 1989a, Carbon-14 in methane sources and in atmospheric methane: The contribution from fossil carbon, Science 245, 286–290.
Wahlen, M., Deck, B., Henry, R., Tanaka, N., Shemesh, A., Fairbanks, R., and Broecker, W., 1989b, Profiles of δ13C of CH4 from the lower stratosphere, EOS, Trans. Amer. Geophys. Union 70 (No. 43), 1017.
Wahlen, M., Tanaka, N., Henry, R., Deck, B., Broecker, W., Shemesh, A., and Fairbanks, R., 1990, 13C, D and 14C in Methane, in Rep. to Congress and the EPA on NASA Upper Atmos. Res. Prog., NASA, Washington DC, pp. 324–325.
Wahlen, M., 1993, The global methane cycle, Annu. Rev. Earth and Planet Sci. 21, 407–426.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tanaka, N., Xiao, Y. & Lasaga, A.C. Ab initio study on carbon Kinetic Isotope Effect (KIE) in the reaction of CH4+Cl•. J Atmos Chem 23, 37–49 (1996). https://doi.org/10.1007/BF00058703
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00058703