Abstract
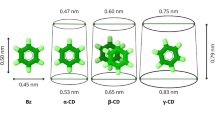
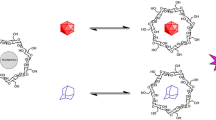
The formation and structure of inclusion complexes of 2,6- and 2,9-substituted bicyclo[3.3.1]nonanes withα- andβ-cyclodextrin (CD) has been investigated by high-resolution1H NMR spectroscopy.α- andβ-CD were found to form 1:1 inclusion complexes and the binding constants were estimated from titration studies. 2D ROESY experiments provided insight into the structure of the complexes.
Similar content being viewed by others
References
J.M. Lehn:Angew. Chem. 100, 91 (1988); G. Wenz:Angew. Chem. Int. Ed. Engl. 33, 803 (1994).
B. Perly, F. Djedani, and P. Berthault:New Trends in Cyclodextrins and Derivatives; Editions de Sante, Paris, 1991.
M.E. Shortreed, S. Wylie, and D.H. Macartney: Inorg Chem. 32, 1824 (1993), and ref. 18–20 cited therein.
R. Bishop and I.G. Dance:Top. Curr. Chem. 149, 137 (1988).
E. Butkus, J. Malinauskiene, and P. Kadziauskas:Z Chem. 20, 103 (1980).
U. Berg, E. Butkus, and A. Stoncius:J. Chem. Soc. Perkin Trans. 2, 97 (1995).
A. Bax and D.G. Davis:J. Magn. Reson. 63, 207 (1985).
D. Marion and K. Wüthrich:Biochem. Biophys. Res. Commun. 113, 967 (1983).
(a) H.A. Benesi and J.H. Hildebrand:J. Am. Chem. Soc. 71, 2703 (1949); (b) O. Bekkers, J.J. Kettenes-van den Bosch, S.P. van Helden, D. Seijkens, J.H. Beijnen, A. Bult, and W.J.M. Underberg:J. Incl. Phenom. 11, 185 (1991).
S. Andini, G. Castrunovo, V. Elia, and E. Gallota:Carbohydr. Res. 217, 87 (1991).
J. Feeney, J.G. Batchelor, J.P. Albrand, and G.C.K. Roberts:J. Magn. Reson. 33, 519 (1979).
D. Neuhaus, and M. Williamson, in:The Nuclear Overhauser Effect in structural and conformational analysis, VCH Publishers, Inc., New York, 1989.
C. Jaime, J. Redondo, F. Sanchez-Ferrando, and A. Virgili:J. Org. Chem. 55, 4772 (1990).
U. Berg, M. fGustavsson, and N. Åström:J. Am. Chem. Soc. 117, 2114 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Butkus, E., Martins, J.C. & Berg, U. 1H NMR spectroscopic study of the interaction between cyclodextrins and bicyclo[3.3.1]nonanes. J Incl Phenom Macrocycl Chem 26, 209–218 (1996). https://doi.org/10.1007/BF01053539
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01053539