Abstract
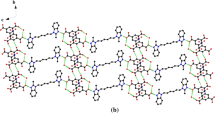
5H-Dibenzo[a, d]cyclohepten-5-ol1 can undergo Ritter reaction with acetonitrile and sulfuric acid to afford either the acetamide derivative2 or the multicyclic amide3 depending on the conditions used. The X-ray structure of the inclusion compound of3 with benzene is reported here and analysed in structural terms. This material [(C19H18N2O)−(C6H6),Cc,a=10.694(5),b=22.843(5),c=9.901(4) Å,β=124.02(2)°,Z=4,R=0.054] has molecules of3 linked by −N−H⋯O=C intermolecular hydrogen bonds to form parallel chains alongc. Additional inter-host stabilisation is achieved by face-face interactions involving one of the two benzo rings of3. A hydrogen atom of the other host benzo group participates in an edge-face interaction with the benzene guest molecule to produce the inclusion compound. Benzene⋯benzene inter-guest interactions provide a further, but minor, contribution to the net stability of the structure.
Similar content being viewed by others
References
J.L. Atwood, J.E.D. Davies, and D.D. MacNicol (Eds.):Inclusion Compounds, Vols. 1–3, Academic Press, London (1984); Vols. 4–5, Oxford University Press, Oxford (1991).
For example: F. Toda, K. Tanaka, and M. Yagi:J. Chem. Soc., Perkin Trans. 1 1215 (1990); F. Toda, inInclusion Compounds, Vol. 4, Ref. 1, above, Ch. 4, pp. 129–130 and references therein.
For example: I. Csöregh, S. Finge, and E. Weber:Bull. Chem. Soc. Jpn. 64, 1971 (1991); E. Weber: inInclusion Compounds, Vol. 4, Ref. 1, above, Ch. 5, pp. 188–262 and references therein.
For example: W.D. Ollis, and J.F. Stoddart: inInclusion Compounds, Vol. 3, Ref. 1, above, 1984, Ch. 6, pp. 169–205 and references therein.
For Part IX see: K.C. Pich, R. Bishop, D.C. Craig, and M.L. Scudder:Aust. J. Chem. 47, 837 (1994).
L.I. Krimen and D.J. Cota:Org. React. (N.Y.) 17, 213 (1969).
R. Bishop:Compr. Org. Synth. 6, 261 (1991).
R. Bishop, G. Burgess, D.C. Craig, I.G. Dance, T. Lipari and M.L. Scudder:J. Incl. Phenom. 10, 431 (1991).
K.C. Pich, R. Bishop, D.C. Craig, and M.L. Scudder:J. Incl. Phenom. 18, 149 (1994).
P. Main: MULTAN 80, University of York, England (1980).
A.D. Rae: RAELS.A Comprehensive Constrained Least Squares Refinement Program, University of New South Wales (1989).
J.A. Ibers and W.C. Hamilton (Eds.):International Tables for X-Ray Crystallography, Vol. 4, Kynoch Press Birmingham (1974). Distributed by Kluwer Academic Publishers, Dordrecht, The Netherlands.
C.K. Johnson: ORTEP-II, Oak Ridge National Laboratory, Tennessee, U.S.A. (1976).
T.R. Lamanec, D.R. Bender, A.M. DeMarco, S. Karady, R.A. Reamer and L.M. Weinstock:J. Org. Chem. 53, 1768 (1988).
G.R. Desiraju:Crystal Engineering — The Design of Molecular Solids, Elsevier, Amsterdam (1989).
Author information
Authors and Affiliations
Additional information
Supplementary Data relating to this article are deposited with the British Library as supplementary publication No. SUP 82189 (10 pages).
Rights and permissions
About this article
Cite this article
Djaidi, D., Bishop, R., Craig, D.C. et al. Ritter reactions. X. Structure of a new multicyclic amide-benzene inclusion compound. J Incl Phenom Macrocycl Chem 20, 363–372 (1994). https://doi.org/10.1007/BF00708880
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00708880