Abstract
We report the synthesis of the first calix[4]arene constrained to a 1,3-alternate conformation by one crown ether and one di-aza-benzo crown ether bridgings. Preliminary binding properties are also given.
Similar content being viewed by others
References
C. D. Gutsche:Calixarenes, Monographs in Supramolecular Chemistry, J. F. Stoddart (Ed.), The Royal Society of Chemistry, London (1989).
J. Vicens and V. Böhmer:Calixarenes: A Versatile Class of Macrocyclic Compounds, Kluwer Academic Publishers, Dordrecht (1991).
A. Ikeda and S. Shinkai:J. Am. Chem. Soc. 116, 3102 (1994).
C. Hill, J.-F. Dozol, V. Lamare, H. Rouquette, B. Tournois, J. Vicens, Z. Asfari, C. Bressot, R. Ungaro and A. Casnati:J. Incl. Phenom. 19, 399 (1994).
H. Yamamoto, T. Sakaki, and S. Shinkai:Chem. Lett. 469 (1994).
E. Ghidini, F. Ugozzoli, R. Ungaro, S. Harkema, A. A. El-Fald, and D. N. Reinhoudt:J. Am. Chem. Soc. 112, 6979 (1990).
Z. Asfari, J. M. Harrowfield, A. N. Sobolev, and J. Vicens:Aust. J. Chem. 47, 757 (1994).
S. Wenger, Z. Asfari, and J. Vicens:Tetrahedron Lett. 8369 (1994).
Z. Asfari, S. Pappalardo, and J. Vicens:J. Incl. Phenom. 14, 189 (1992).
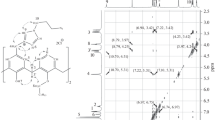
Preparation of (1): In a 1000 mL 2-necked round bottom flask, a solution of calix[4]arene (4.245 g, 10.00 mmoles) and potassium carbonate (1.451 g. 10.50 mmoles) in acetonitrile (700 mL) was stirred at r. t. overnight. A solution of tetraethylene glycol ditosylate (5.026 g, 10.00 mmoles) in acetonitrile (20 mL) was added in one time and refluxed for 8 days. After evaporation to dryness under reduced pressure, the residue was dissolved in chloroform and the potassium carbonate neutralised with 1N HCl. The organic layer was dried over Na2SO4 and concentrated to obtain a yellow solid (6.571 g). Pure product (1) (1.556 g, 27%) was obtained as a white solid after chromatography (SiO2, Bio-Rad, Bio Sil 40–63, CH2Cl2/acetone 95/5). Mp. 263–264°C.1H-NMR (CDCl3, 200 MHz, 25°C): δ 3.37 and 4.43 (AB system,J=13Hz, 8H, CH2), 3.86 (t,J=4.8 Hz, 4H, CH2), 3.96 (t,J=4.8 Hz, 4H, CH2), 4.10 (s, 8H, CH2), 6.67–7.10 (m, 12H, arom.), 7.74 (s, 2H, OH). Positive ion FAB,m/z=582.2 (M+, 100%).Found: H 6.47, C 74.26,Calcd. for C36H38O7: H 6.57, C 74.21.Preparation of (3): In a 250 mL 2-necked round bottom flask, a solution of (1) (1.165 g, 2.00 mmoles) and potassium carbonate (1.382 g, 10.10 mmoles) in acetonitrile (150 mL) was stirred at r. t. for 4 h. A solution of (2) (2.159 g, 2.00 mmoles) in acetonitrile (15 mL) was added dropwise and refluxed for 2 days. After evaporation to dryness under reduced pressure, the residue was dissolved in chloroform and the potassium carbonate neutralised with 1N HCl. The organic layer was dried over Na2SO4 and concentrated to obtain a beige solid (3.019 g). Pure product (3) (0.525 g, 20%) was obtained as a white solid after chromatography (SiO2, Bio-Rad, Bio Sil 40–63, CH2Cl2/MeOH/acetone 90/5/5). Mp. 92–93°C.1H-NMR (CDCl3, 200 MHz, 25°C): δ 1.50–1.57 (m, 2H, CH2), 2.41 (s, 6H, CH3), 2.98 (t,J=6.9 Hz, 4H, CH2), 3.13 (t,J=5.00 Hz, 8H, CH2), 3.39–3.93 (m, 24H, CH2), 3.86 (s, 8H, CH2), 4.25 (s, 4H, CH2), 6.75–7.64 (m, 28H, arom.). Positive ion FAB,m/z=1317.4 (M+, 58%), 1161.4 (M+−C7H7O2S1, 100%).Found: H 6.37, C 68.13;Calcd. for C75H84O15 N2S2: H 6.43, C. 68.37.
C. Alfieri, E. Dradi, A. Pochini, R. Ungaro, and G. D. Andreetti:J. Chem. Soc., Chem. Commun. 1075 (1983).
Preparation of the 1: 1 complex (3)-K +Pic−:ligand (3) (0.015 g, 0.011 mmoles) and solid potassium picrate (0.016 g, 0.057 mmoles) were stirred for 24 h. The unreacted K+Pic− was filtered off and the filtrate evaporated to dryness to give a yellow solid. Mp=120–121°C. Quantitatively yield.1H-NMR (CDCl3, 200 MHz, 25°C): δ 1.25 (s large, 2H, CH2), 2.41 (s, 6H, CH3), 2.95 (t,J=6.9 Hz, 4H, CH2), 3.56 (t,J=5.00 Hz, 8H, CH2), 3.66–4.10 (m, 24H, CH2), 3.80 (s, 8H, CH2), 4.28 (s, 4H, CH2), 6.71–7.62 (m, 28H, arom.), 8.79 (s, 2H, arom.). Positive ion FAB,m/z=1355.0 (M++K+, 100%).Found: H 5.70, C 61.30;Calcd. for C81H86O22N5S2K1: H 5.47, C 61.39.Preparation of the 1:1 complex (3)-NH +4 Pic −:ligand (3) (0.015 g, 0.011 mmoles); ammonium picrate (0.015 g, 0.057 mmoles); 2 h. Mp=85–86°C. Quantitative yield.1H-NMR (CDCl3, 200 MHz, 25°C): δ 1.36 (s large, 2H, CH2), 2.41 (s, 6H, CH3), 2.95 (t,J=6.9 Hz, 4H, CH2), 3.51 (t,J=5.00 Hz, 8H, CH2), 3.67–4.10 (m, 24H, CH2), 3.82 (s, 8H, CH2), 4.28 (s, 4H, CH2), 6.69–7.61 (m, 28H, arom.), 8.79 (s, 2H, arom.). Positive ion FAB,m/z=1334.7 (M++NH +4 , 34%).Found: H 5.88, C 60.16;Calcd. for C81H90O22N6S2·3H2O: H 5.98, C 60.14.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wenger, S., Asfari, Z. & Vicens, J. Synthesis of an unsymmetrically doubly bridged calix[4]arene in the 1,3-alternate conformation. J Incl Phenom Macrocycl Chem 20, 293–296 (1994). https://doi.org/10.1007/BF00708875
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00708875