Abstract
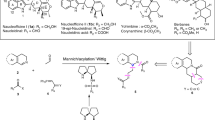
[2.2]Cyclic Tröger base2 was synthesized by the condensation of 1,2-bis (4-aminopheyl) ethane with paraformaldehyde under acidic condition in 43.8% yield. [2.2]Cyclic Tröger base2 was separated into meso form and racemate by fractional crystallization or using HPLC. Resolution of the racemate into its optical antipodes by passing the racemate through an activated D-(+)-lactose column was partially succeeded.
Similar content being viewed by others
References and Notes
For reviews of host-guest chemistry; “Host Guest Complex Chemistry I” ed. by F. Vögtle, Springer-Verlag, Berlin Heidelberg, 1981.
“Host Guest Complex Chemistry II” ed. by F. Vögtle, Springer-Verlag, Berlin Heidelberg 1982.
D. J. Cram,Science,219, 1177 (1983).
J. Tröger,J. prakt. Chem., [2]36, 227 (1887).
1,2′-Methylene-3-(4′-tolyl)-6-methyl-1,2,3,4-tetrahydroquinazoline.
Tröger base can be prepared merely by allowing a mixture of formaldehyde, p-toluidine and concentrated HCl in acetic acid to stand for several days. Structures proposed by Tröger and by later workers was incorrect, but Spielman8), established the structure by an unequivocal synthesis.
M. A. Spielman,J. Am. Chem. Soc.,57, 583 (1935).
V. Prelog and P. Wieland,Helv. Chim. Acta,27, 1127 (1944).
O. Červinka, A. Fábryová, and V. Novák,Tetrahedron Lett., 5375 (1966). Červinka et al. deduced from the empirical comparison of the ORD spectra of (+)-Tröger base and (−)-argemonine that the absolute configuration in it was 1S, 5S, but later Mason11a, 11b) et al. analyzed the CD curve of (+)-and (−)-Tröger base and reversed the configuration as shown in Scheme 1.
S. F. Mason, G. W. Vane, K. Schofield, R. J. Wells, and J. S. Whitehurst,J. Chem. Soc., (B), 553 (1967).
S. F. Mason, K. Schofield, R. J. Wells, J. S. Whitehurst, and G. W. Vane,Tetrahedron Lett., 137 (1967).
Author information
Authors and Affiliations
Additional information
The trivial name “[2.2]cyclic Tröger base” is used because of the cumbersome nomenclature.
10,12,25,32-Tetraazanonacyclo[19.7.7.14,8.114,18.125,32.07,12.07,12.010,15.-024,34.027,31] octatriaconta-1(29),4,6,8(36),14,16,18(37),21,23,27,-30,34-dodecaene.
Rights and permissions
About this article
Cite this article
Fukae, M., Inazu, T. Synthesis of macrocycles having tröger base skeletons “[2.2]cyclic tröger base”. Journal of Inclusion Phenomena 2, 223–229 (1984). https://doi.org/10.1007/BF00663260
Issue Date:
DOI: https://doi.org/10.1007/BF00663260