Abstract
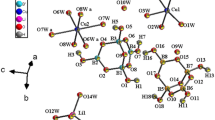
Single-crystal X-ray structure analyses of N(nPr)4[B5O6(OH)4][B(OH)3]2,1, and N(nBu)4 [B5O6(OH)4][B(OH)3]2,2, reveal that these materials are novel clathrates, the isotypic host structures of which are three-dimensional assemblies of hydrogen-bonded [B5O6(OH)4]− ionsand B(OH)3 molecules. The assembly of only the pentaborate anions is a distorted (i.e., along [102] elongated) fourconnected diamond-related network. The N(nPr) +4 and N(nBu) +4 ions are trapped within the complex three-dimensional channel systems of the host frameworks. Both1 and2 crystallize monoclinically with space groupP21/c andZ=4. The cell constants are:1:a=13.592(5),b=12.082(2),c=17.355(6) Å, β=106.60(2)° (298K);2:a=13.874(3),b=12.585(1),c=17.588(4) Å, β=107.04(1)° (238 K). The results obtained by both11B and13C MAS NMR spectroscopy are discussed. Thermogravimetric studies under a flowing inert-gas atmosphere suggest that water, stemming from polycondensation of the hydrous borate species, is released from the clathrates at ca. 443 K (1) and 398 K (2) before the decomposition of the organic cations starts at ca. 603 K (1) and 603 K (2).
Similar content being viewed by others
References
O. Ermer:J. Am. Chem. Soc. 110, 3747 (1988)
O. Ermer and L. Lindenberg:Helv. Chim. Acta 74, 825 (1991).
M. Simard, D. Su, and J.D. Wuest:J. Am. Chem. Soc. 113, 4696 (1991).
S.B. Copp, S. Subramanian, and M.J. Zaworotko:J. Am. Chem. Soc. 114, 8719 (1992)
S.B. Copp, S. Subramanian, M.J. Zaworotko:J. Chem. Soc., Chem. Commun. 1078 (1993).
O. Ermer and L. Lindenberg:Chem. Ber. 123, 1111 (1990).
M. Wiebcke, C.C. Freyhardt, J. Felsche, and G. Engelhardt:Z. Naturforsch. 48b, 978 (1993).
G. Heller:J. Inorg. Nucl. Chem. 30, 2743 (1968).
F.H. Allen:Acta Crystallogr. B42, 515 (1986).
SDP-Structure Determination Package, Enraf-Nonius, Delft, The Netherlands (1989).
International Tables for X-ray Crystallography Vol. IV, Kynoch Press, Birmingham (1974). Distributed by Kluwer Academic Publishers, Dordrecht, The Netherlands.
C.K. Johnson:ORTEP II Report ORNL-5138, Oak Ridge National Laboratory, TN, USA (1976).
E. Keller:SCHAKAL92-A FORTRAN Program for the Graphical Representation of Molecular and Crystallographic Models, University of Freiburg (1992).
M. Gajhede, S. Larsen, and S. Rettrup:Acta Crystallogr. B42, 545 (1986).
A.F. Wells:Structural Inorganic Chemistry 5th Ed., Clarendon Press, Oxford, pp. 1072 (1984).
R.W. Alder, C.M. Maunder, and A.G. Orpen:Tetrahedron Lett. 31, 6717 (1990).
D.M.P. Mingos and A.L. Rohl:J. Chem. Soc., Dalton Trans. 3419 (1991)
A. Gavezzotti:J. Am. Chem. Soc. 103, 5220 (1983).
G.L. Turner, K.A. Smith, R.J. Kirkpatrick, and E. Oldfield:J. Magn. Reson. 67, 544 (1986)
D. Müller, A.-R. Grimmer, U. Timper, G. Heller, M. Shakibaie-Moghadam:Z. Anorg. Allg. Chem. 619, 1262 (1993).
G. Engelhardt and D. Michel:High-Resolution Solid-State NMR of Silicates and Zeolites, John Wiley & Sons, Chichester, p. 350 (1987).
J.-M. Chezeau, L. Delmotte, J.-L. Guth, and M. Soulard:Zeolites 9, 78 (1989)
N. Dumont, Z. Gabelica, E.G. Derouane, and L.B. McCusker:Microporous Mater. 1, 149 (1993)
J.B. Nagy, Z. Gabelica, and E.G. Derouane:Zeolites 3, 43 (1983).
C. Wan and S. Ghose:Am. Mineral. 68, 604 (1983)
P.C. Burns and F.C. Hawthorne:Acta Crystallogr. C50, 653 (1994).
S. Menchetti, C. Sabelli, and R. Trosti-Ferroni:Acta Crystallogr. B38, 2987 (1982).
J. Krogh-Moe:Acta Crystallogr. B28, 168 (1972).
Author information
Authors and Affiliations
Additional information
Author for correspondence.
Supplementary Data relating to this article are deposited with the British Library as supplementary publication No. SUP 82172 (82 pages).
Rights and permissions
About this article
Cite this article
Freyhardt, C.C., Wiebcke, M., Felsche, J. et al. N(nPr)4[B5O6(OH)4][B(OH)3]2 and N(nBu)4[B5O6(OH)4][B(OH)3]2: Clathrates with a diamondoid arrangement of hydrogen-bonded pentaborate anions. Journal of Inclusion Phenomena and Molecular Recognition in Chemistry 18, 161–175 (1994). https://doi.org/10.1007/BF00705819
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00705819