Abstract
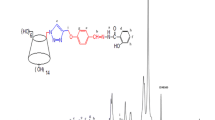
The paper reports the synthesis of aβ-cyclodextrin (β-CD) derivative (1) functionalized with a ligand subunit at the secondary-hydroxyl rim. The ligand subunit is 2-hydroxymethyl-6-thiomethyl pyridine connected to the macrocycle via a thioether bond. In the presence of Cu(II) ions1 accelerates the cleavage of thep-nitrophenyl esters of picolinic acid (PNPP), quinaldic acid (PNPQ) and its 6-phenyl derivative (PNPQPh) via the nucleophilic attack of the hydroxyl of the pyridine subunit. However, theβ-CD derivative is less effective than the ligand 2-hydroxymethyl-6-methylthiomethyl pyridine (2), indicating no cooperation between the hydrophobic and metal ion recognition sites. However, in the case of PNPQPh, the observed rate constants in the presence of Cu(II) ions are close to that of model2 and this suggests we are approaching a binding mode appropriate for taking advantage of the two binding sites of the metal receptor1 · Cu(II). Interestingly, the most reactive derivative with nativeβ-CD is thep-nitrophenyl quinaldate (PNPQ) in accord with its mode of complexation to the macrocycle and the location of the actual nucleophile (one of the secondary hydroxyls ofβ-CD).
Similar content being viewed by others
References
A. P. Croft and R. A. Bartsch:Tetrahedron 38, 1417 (1983).
K. Rama Rao, T. N. Srinivasan, N. Bhanumathi and P. B. Sattur:J. Chem. Soc., Chem. Commun. 10 (1990); H. Ikeda, R. Kojin, C.-J. Yoon, T. Ikeda and F. Toda:Tetrahedron Lett. 29, 311 (1988); H. Ikeda, R. Kojin, C.-J. Yoon, T. Ikeda, and F. Toda:Chem. Lett. 1495 (1987); F. Cramer and G. Mackensen:Chem. Ber. 103, 2138 (1970).
R. L. VanEtten, J. F. Sebastian, G. A. Clowers, and M. Bender:J. Am. Chem. Soc. 89, 3242 (1967).
J. Chin,Acc. Chem. Res. 24, 145 (1991): P. Scrimin, P. Tecilla, U. Tonellato, and N. Vignaga:J. Chem. Soc., Chem. Commun. 449 (1991).
R. Breslow and L. E. Overman:J. Am. Chem. Soc. 92, 1075 (1970).
I. Tabushi and Y. Kuroda:J. Am. Chem. Soc. 106, 4580 (1984); I. Tabushi, Y. Kuroda, and T. Mizutani:Tetrahedron 40, 545 (1984); I. Tabushi, N. Shimizu, T. Sugimoto, M. Shiozuka, and K. Yamamura:J. Am. Chem. Soc. 99, 7100 (1977).
I. Wilnder and Z. Goren:J. Chem. Soc., Chem. Commun. 1469 (1983).
J. Franke, T. Merz, H.-W. Losensky, M. Muller, U. Werner, and F. Vögtle:J. Incl. Phenom. 3, 469 (1985).
M. I. Rosenthal and A. W. Czarnik:J. Incl. Phenom. 10, 119 (1991); E. U. Akkaya and A. W. Czarnik:J. Am. Chem. Soc. 110, 8553 (1988).
H.-J. Schneider and F. Xiao:J. Chem. Soc. Perkin Trans. 2, 387 (1992).
R. P. Bonomo, V. Cucinotta, F. D'Alessandro, G. Impellizzeri, G. Maccarone, G. Vecchio, and E. Rizzarelli:Inorg. Chem. 30, 2708 (1991); V. Cucinotta, F. D'Alessandro, G. Impellizzeri, G. Pappalardo, E. Rizzarelli, and G. Vecchio:J. Chem. Soc., Chem. Commun. 293 (1991).
R. Fornasier, P. Scrimin, P. Tecilla, and U. Tonellato:J. Am. Chem. Soc. 111, 224 (1989).
A. I. Vogel:A Textbook of Quantitative Inorganic Analysis, Longman, London (1961).
D. S. Sigman and C. T. Jorgensen:J. Am. Chem. Soc. 94, 1724 (1972).
T. R. Kelly, T. E. Schmidt, and J. C. Haggerty:Synthesis 544 (1972).
R. J. Leatherbarrows:Enzfitter, Elsevier, Amsterdam (1987).
P. Scrimin, P. Tecilla, and U. Tonellato:J. Org. Chem. 56, 161 (1991).
A. Ueno and R. Breslow:Tetrahedron Lett. 23, 3451 (1982); R. Breslow, A. W. Czarnik, M. Lauer, R. Leppkes, J. Winkler, and S. Zimmerman:J. Am. Chem. Soc. 108, 1969 (1986).
E. Borrione, M. Prato, G. Scorrano, M. Stivanello, and V. Lucchini:J. Heterocyclic Chem. 25, 1831 (1988); M. Prato, V. Lucchini, G. Scorrano, M. Stivanello, and P. Tecilla:Gazz. Chim. Ital. 117, 325 (1987).
A. R. Amundsen, J. Whelan, and B. Bosnich:J. Am. Chem. Soc. 99, 6730 (1977).
P. Scrimin, P. Tecilla, U. Tonellato, and T. Vendrame:J. Org. Chem. 54, 5988 (1989).
T. H. Fife and T. J. Przystas:J. Am. Chem. Soc. 107, 1041 (1985).
H.-J. Schneider, T. Blatter, and S. Simova:J. Am. Chem. Soc. 113, 1996 (1991).
D. B. Smithrud and F. Diederich:J. Am. Chem. Soc. 112, 339 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fornasier, R., Scarpa, E., Scrimin, P. et al. A new ligand-functionalizedβ-cyclodextrin as a esterolytic reagent at neutral pH. J Incl Phenom Macrocycl Chem 14, 205–215 (1992). https://doi.org/10.1007/BF01045981
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01045981