Abstract
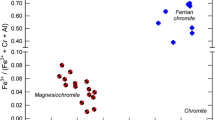
The variety of coordination numbers, symmetries, distortions and ligand environments in thermally-stable iron-bearing minerals provide wide ranges of chemical shift (δ) and quadrupole splitting (δ) parameters, which serve to characterize the crystal chemistries and site occupancies of Fe2+ and Fe3+ ions in minerals of terrestrial and extraterrestrial origins. Correlations between ferrous and ferric chemical shifts enable thermally-induced electron delocalization behavior in mixed-valence Fe2+-Fe3+ minerals to be identified, while chemical shift versus quadrupole splitting correlations serve to identify nanophase ferric oxides and oxyhydroxides in oxidized minerals and in meteorites subjected to aqueous oxidation before and after they arrived on Earth.
Similar content being viewed by others
References
G.M. Bancroft, R.G. Burns et al., Nature 212(1966)913; 213(1967)1221; Am. Miner. 52(1967)1009 and 1278; Earth Planet. Sci. Lett. 3(1967)125; Geochim. Cosmochim. Acta 31(1967)2219; 32(1868)547; Min. Soc. Am. Spec. Pap. 2(1969)137.
G.M. Bancroft and R.G. Burns, Acta Cryst. 22(1967)934; Geochim. Cosmochim. Acta 32(1968)547.
G.M. Bancroft and R.G. Burns, Miner. Mag.,1966 IMA Volume (1968)170.
G.M. Bancroft,Mössbauer Spectroscopy: An Introduction for Inorganic Chemists and Geochemists (McGraw-Hill, 1973).
R.G. Burns and T.C. Solberg, in:Spectroscopic Correlations for Minerals and Their Surfaces, eds. L.M. Coyne, S.W.S. McKeever and D.F. Blake, chap. 14, ACS Symp. Ser. 415(1990)262.
R.G. Burns, in:Mixed Valency Systems: Applications in Chemistry, Physics and Biology, ed. K. Prassides (Plenum Press); NATO ASI-C Ser. 343(1992)175.
W. Kündig et al., Phys. Rev. 142(1966)327; Czech. J. Phys. B17(1967)467.
R.V. Morris et al., J. Geophys. Res. 90(1985)3126; 94(1989)2760.
K.S. Bartels and R.G. Burns, Lunar Planet. Sci. 20(1989)44.
D.W. Straub, R.G. Burns and S.F. Pratt, J. Geophys. Res. 96(1991)18819.
J.L. Bishop, C.M. Pieters and R.G. Burns, Lunar Planet. Sci. 23(1992)111; 24(1993)115; Geochim. Cosmochim. Acta 57(1993), in press.
T.C. Solberg and R.G. Burns,Proc. 19th Lunar Planet. Sci. Conf. (1989) p. 313.
R.G. Burns and S.L. Martinez,Proc. 21st Lunar Planet. Sci. Conf. (1991) p. 331.
D.S. Fisher and R.G. Burns, Lunar Planet. Sci. 22(1991)389; 24(1993)489.
R. Ostertag, G. Amthauer, H. Rager and H.Y. McSween, Jr., Earth Planet. Sci. Lett. 67(1984)162.
R.G. Burns, in:Remote Geochemical Analysis: Elemental and Mineralogical Composition, eds. C. Pieters and P.A.J. Englert (Cambridge University Press, 1993) chap. 26.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burns, R.G. Mineral Mössbauer spectroscopy: Correlations between chemical shift and quadrupole splitting parameters. Hyperfine Interact 91, 739–745 (1994). https://doi.org/10.1007/BF02064600
Issue Date:
DOI: https://doi.org/10.1007/BF02064600