Abstract
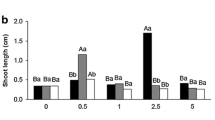
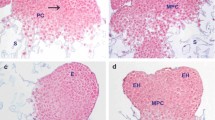
In vitro formation of roots and somatic embryos is obtained from cotyledon explants of a Spindle tree (Euonymus europaeus L.) cultured on two different media: a medium inducing callus formation and the production of roots, and a medium inducing callus formation, root and somatic embryo production. We studied the effects of α-difluoromethylornithine (DFMO), a specific, irreversible inhibitor of ornithine decarboxylase (ODC) on root and somatic embryo production, growth and titers of putrescine in Euonymus explants and explant-derived calli. Early changes in putrescine levels were detected in both cultures before the visible emergence of roots or somatic embryos. DFMO rapidly inhibited putrescine accumulation and growth in non-embryogenic calli and highly stimulated rooting activity. DFMO partially inhibited putrescine accumulation in embryogenic calli. This inhibition had no effects on callus growth but significantly reduced the time of emergence of roots and highly stimulated somatic embryo production. The relationship among putrescine, putrescine metabolism, growth, root and somatic embryo formation is discussed.
Similar content being viewed by others
References
Burtin D, Martin-Tanguy J, Paynot M, Carre M and Rossin N (1990) Polyamines, hydroxycinnamoyl putrescines and root formation in leaf explants of tobacco cultivated in vitro. Plant Physiol 93: 1348–1404
Desai HV and Mehta AR (1985) Changes in polyamine levels during shoot formation, root formation and callus induction in cultured Passiflora leaf discs. J Plant Physiol 119: 45–53
El Hadrami I, Michaux-Ferrières N, Carron MP and d'Auzac J (1990) Polyamines, a possible limiting factor in embryogenesis of Hevea brasiliensis. C R Acad Sci Paris Série III: 205–211
Evans PT and Malmberg RL (1989) Do polyamines have roles in plant development? Ann Rev Plant Physiol Plant Mol Biol 40: 235–269
Feirer RP, Mignon G and Litvay JD (1984) Arginine decarboxylase and polyamines required for embryogenesis in the wild carrot. Science 223: 1433–1435
Fienberg AA, Choi JH, Lubich WP and Sung ZR (1984) Development regulation of polyamine metabolism in growth and differentiation of carrot culture. Planta 161: 532–539
Flores HE and Galston AW (1982) Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol 69: 701–706
Galston AW (1983) Polyamines as modulators of plant development. Bioscience 33: 102–109
Galston AW and Kaur-Sahwney R (1987) Polyamines as endogenous growth regulators. In: Davies PJ (ed) Plant Hormones and their Role in Plant Growth and Development, pp 280–295. Boston: Martinus Nijhof
Kaur-Sahwney R, Tiburcio AE and Galston AW (1986) Polyamine-mediated control of organogenesis in tobacco thin layers. Plant Physiol 80: S-193
Martin-Tanguy J, Martin C, Paynot M and Rossin N (1988) Effect of hormone treatment on growth bud formation and free amine and hydroxycinnamoyl putrescine levels in leaf explant of Nicotiana tabacum cultivated in vitro. Plant Physiol 88: 600–604
Montague MJ, Koppenbrink JW and Jaworski EG (1978) Polyamine metabolism in embryogenic cells of Daucus carota, 1. Changes in intracellular content and rates of synthesis. Plant Physiol 62: 430–433
Murashige T and Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–597
Pegg AE and MacCann PP (1982) Polyamine metabolism and function. Am J Physiol 243: C212-C221
Roberts DR, Dumbroff EB and Thompson JE (1985) Exogenous polyamines alter membrane fluidity in bean leaves. A basic potential misinterpretation of their true physiological role. Planta 167: 395–401
Robie CA and Minocha SC (1989) Polyamines and somatic embryogenesis in carrot, 1. The effects of difluoromethylornithine and difluoromethylarginine. Plant Sci 65: 45–54
Slocum RD, Kaur-Sawhney R and Galston AW (1984) The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys 235: 283–303
Smith MA and Davies PJ (1985) Separation and quantification of polyamines in plant tissue by high performance liquid chromatography of their dansyl derivatives. Plant Physiol 78: 89–91
Smith TA (1985) Polyamines. Ann Rev Physiol 36: 117–143
Torrigiani P, Altamura MM, Pasqua G, Monacelli B, Serrafini-Fracassini D and Bagni N (1987) Free and conjugated polyamines during de novo floral and vegetative bud formation in thin layers of tobacco. Physiol Plant 70: 453–460
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bonneau, L., Beranger-Novat, N., Monin, J. et al. Stimulation of root and somatic embryo production in Euonymus europaeus L. by an inhibitor of polyamine biosynthesis. Plant Growth Regul 16, 5–10 (1995). https://doi.org/10.1007/BF00040501
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00040501