Abstract
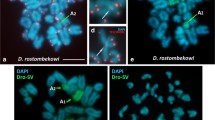
It has been suggested that the karyotype of the marsupials derived from a low diploid number (2n = 14) which originated, through fissions of biarmed chromosomes, the karyotypes with a higher 2n. The telomeric sequence (T2AG3)nwas in situhybridized to the chromosomes of Gracilinanus microtarsusand G. emiliae, Micoureus demeraraeand Marmosa murina, species with 2n = 14, in Monodelphissp., M. domestica, M. kunsiand M. brevicaudatawith 2n = 18, and in Lutreolina crassicaudata, Didelphis albiventris, Chironectes minimus, Philander opossumand P. frenata, all of them with 2n = 22. The probe hybridization occurred in the telomeric regions of both arms, short and long, of all chromosomes of the complement of all individuals of all species analysed. However, in some pairs of the karyotypes of Gracilinanus microtarsusand Micoureus demerarae(with 2n = 14), and in Monodelphissp., M. domestica, M. kunsiand M. brevicaudata(2n = 18) ectopic signs of hybridization were detected proximal to the centromeres, suggesting the retention of this telomeric sequence in the centromeric regions of some chromosomes of these species. Based on these results, it is proposed that the karyotype of marsupials evolved from a 2n = 22 to a 2n = 14, by means of chromosomal fusions.
Similar content being viewed by others
References
Baker, R.J.,M.W. Haiduk, L.W. Robbins, A. Candena & B.F. Koop, 1982. Chromosomal studies of American bats and their systematic implications, pp. 303–327 in Mammalian Biology in South America, edited by M.A. Mares & H.H. Genoways. Special Publications Series, Pymatuning Laboratory of Ecology, University of Pittsburg.
Clarck, M.S. & W.J. Wall, 1996. Speciation in an ancient mammalian group, pp. 246–254 in Chromosomes – The complex Code. Chapman and Hall, London.
Clemens, W.A, 1968. Origin and early evolution of marsupials. Evolution 22: 1–18.
Fonseca, G.A.B., G. Herrmann, Y.R.L. Leite, R.A. Mittermeier, A.B. Rylands & J.L. Patton, 1996. Lista anotada dos mamíferos do Brasil. Occas. Papers Conserv. Biol. 4: 1–38.
Garagna, S., D. Broccoli, C.A. Redi, J.B. Searle, H.J. Cooke & E. Capanna, 1995. Robertsonian metacentrics of the house mouse lose telomeric sequences but retain some minor satellite DNA in the pericentromeric area. Chromosoma 103: 685–692.
Gardner, A.L., 1993. Order Didelphimorphia, pp. 15–23 in Mammals Species of the World: A taxonomic and geographic reference, edited by D.E. Wilson & D.M. Reeder. Smithsonian Institution, Washington.
Go, Y., G. Rakotoarisoa, Y. Kawamoto, A. Randrianjafy, N. Koyama & H. Hirai, 2000. PRINS analysis of the telomeric sequence in seven lemurs. Chromos. Res. 8(1): 57–65.
Hayman, D.L., 1990. Marsupial cytogenetics. Aust. J. Zool. 37: 331–349.
Hope, R.M., S. Cooper & B. Wainwright, 1990. Globin macromolecular sequences in marsupials and monotremes, pp. 147–172 in Mammals from Pouches and Eggs: Genetics, Breeding and Evolution of Marsupials and Monotremes, edited by J.A.M. Graves, R.M. Hope & D.W. Cooper. CSIRO, Melbourne.
Lee, C., R. Sasi & C.C. Lin, 1993. Intersticial localization of telomeric DNA sequences in the Indian muntjac chromosomes: further evidence for tandem chromosome fusions in the karyotypic evolution of the Asian muntjacs. Cytogenet. Cell Genet. 63: 156–159.
Matthey, R., 1973. The chromosome formulae of eutherian mammals, pp. 531–616 in Cytotaxonomy and Vertebrate Evolution, edited by A.B. Chiarelli & E. Capanna. Academic Press, New York/London.
Metcalfe, C.J., M.D.B. Eldridge, L.R. McQuade & P.G. Johnston, 1997. Mapping the distribution of the telomeric sequence (T2AG3)nin rock-wallabies, Petrogale(Marsupialia: Macropodidae), by fluorescence in situhybridization. I. The penicillatacomplex. Cytogenet. Cell Genet. 78: 74–80.
Metcalfe, C.J., M.D.B. Eldridge, R. Toder & P.G. Johnston, 1998. Mapping the distribution of the telomeric sequence (T2AG3)nin theMacropodoidea (Marsupialia), by fluorescence in situhybridization. I. The swamp wallaby, Wallabia bicolor. Chromos. Res. 6: 603–610.
Meyne, J., R.J. Baker, H.H. Hobart, T.C. Hsu, O.A. Ryder, O.G. Ward, J.E. Wiley, D.H. Wurster-Hill, T.L. Yates & R.K. Moysis, 1990. Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequences in vertebrate chromosomes. Chromosoma 99: 3–10.
Palma, R.E. & T.L. Yates, 1996. The chromosomes of bolivian Didelphid marsupials. Occas. Pappers Mus. Texas Tech Univ. 162: 1–20.
Qumsiyeh, M.B., J.L. Coate, J.A. Peppers & M.L. Kennedy, 1997. Robertsonian chromosomal rearrangements in short-tailed shrew, Blarina carolinensis, in western Tennessee. Cytogenet. Cell Genet. 76: 153–158.
Reig O.A., A.L. Gardner, N.O. Bianchi & J.L. Patton, 1977. The chromosomes of the Didelphidae (Marsupialia) and their evolutionary significance. Biol. J. Linn. Soc. Lond. 9: 191–216.
Retief, J.D., C. Krajewski, M. Westerman, R.J. Winkfein & G.H. Dixon, 1995. Molecular phylogeny and evolution of marsupial protamine P1 genes. Proc. R. Soc. Lond. B 259: 7–14.
Rofe, R. & D.L. Hayman, 1985. G-banding evidence for a conserved complement in the Marsupialia. Cytogenet. Cell Genet. 39: 40–50.
Schmid, M., W. Feichtinger, I. Nanda, R. Schakowski, R. Visbal Garcia, J. Manzanilla Puppo & A. Fernandez Badillo, 1994. An extraordinarily low diploid chromosome number in the reptile Gonatodes taniae(Squamata, Gekkonidae). J. Hered. 85: 255–260.
Schubert, I., G. Schriever-Schwemmer, T. Werner & I.D. Adler, 1992. Telomeric signals in Robertsonian fusion and fission chromosomes: implications for the origin of pseudoaneuploidy. Cytogenet. Cell Genet. 59: 6–9.
Sharman, G.B., 1974. Marsupial taxonomy and phylogeny. Austral. Mammal. 1: 137–154.
Svartman, M. & A.M. Vianna-Morgante, 1998. Karyotype evolution of marsupials: from higher to lower diploid numbers. Cytogenet. Cell Genet. 82: 263–266.
Svartman, M. & A.M. Vianna-Morgante, 1999. Comparative analysis in American marsupials: chromosome banding and in situhybridization. Chromos. Res. 7: 267–275.
Vermeesch, J.R., W. De Meurichy, H. Van Den Berghe, P. Marynen & P. Petit, 1996. Differences in the distribution and nature of the interstitial telomeric (TTAGGG)nsequences in the chromosomes of the Giraffidae, okapi (Okapia johnstoni), and giraff (Giraffia camelo pardalis): evidence for ancestral telomeres at the okapi polymorphic rob (4; 26) fusion site. Cytogenet. Cell Genet. 72: 310–315.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carvalho, d.A., Mattevi, M.S. (T2AG3)n Telomeric Sequence Hybridization Suggestive of Centric Fusion in Karyotype Marsupials Evolution. Genetica 108, 205–210 (2000). https://doi.org/10.1023/A:1004157915077
Issue Date:
DOI: https://doi.org/10.1023/A:1004157915077