Abstract
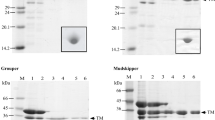
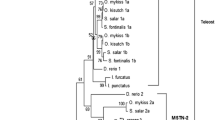
The structural stability of fish myosin depends upon species and temperatures of water in which fish live. Primary, secondary, and quaternary structures of myosin heavy chain (MyHC) from three species of fish living at different temperature ranges have been compared with those of rabbit MyHC in order to investigate the differences in stability. Primary structure of MyHC, although being accessible for warm-water and cold-water fish (carp and walleye pollack), was not available in previous for tropical-water fish literature; so in this study primary structure of MyHC of the tropical-water fish amberjack has been determined by cloning and sequencing its cDNA. The MyHC has 1938 amino acid residues (AA), which are almost as much as as those of carp and walleye pollack. The amberjack MyHC is 91–95% homologous with other fish and rabbit MyHCs. There is a discernible difference between animal species with stable myosin rod (amberjack, carp, and rabbit) and walleye pollack with unstable rod. Stable rod species have a high probability of forming coiled-coil around the COOH-terminal end of the rod, while the pollack has a low coiled-coil formation probability. In addition, the average scores of the coiled-coil for myosin rod were rabbit (1.738) > amberjack (1.691) > carp (1.680) > walleye pollack (1.674) which correlated exactly with the observed stability. The results suggest that coiled-coil forming ability, particularly around the COOH-terminal end, directs structural stability of fish myosin rod.
Similar content being viewed by others
References
Arai, K., Kawamura, K. and Hayashi, C. 1973. The relative thermostabilities of the actomyosin-ATPase from the dorsal muscles of various fish species. Bull. Japan. Soc. Sci. Fish. 39: 1076-1085.
Chomczynski, P. and Sacchi, N. 1987. Single-step method of RNA isolation by acid guanidinum thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162: 156-159.
Connell, J.J. 1961. The relative stabilities of the skeletal muscle myosins of some animals. Biochem. J. 80: 503-509.
Goldspink, G. 1995. Adaptation of fish to different environmental temperature by qualitative and quantitative changes in gene expression. J. Therm. Biol. 20: 167-174.
Holzwarth, G. and Doty, P. 1965. The ultraviolet circular dichroism of polypeptides. J. Am. Chem. Soc. 87: 218-228.
Johnston, I.A., Frearson, N. and Goldspink, G. 1973. The effects of environmental temperature on the properties of myofibrillar adenosine triphosphatase from various species of fish. Biochem. J. 133: 735-738.
Johnston, I.A. and Goldspink, G. 1975. Thermodynamic activation parameters of fish myofibrillar ATPase enzyme and evolutionary adaptaions to temperature. Nature 257: 620-622.
Kakinuma, M., Nakaya, M., Hatanaka, A., Hirayama, Y., Watabe, S., Maeda, K., Ooi, T. and Suzuki, S. 1998. Thermal unfolding of three acclimation temperature-associated isoforms of carp light meromyosin expressed by recombinant DNAs. Biochemistry 37: 6606-6613.
Kato, S. and Konno, K. 1993. Filament-forming domain of carp dorsal myosin rod. J. Biochem. 113: 43-47.
Katoh, N., Uchiyama, H., Tsukamaoto, S. and Arai, K. 1977. A biochemical study on fish myofibrillar ATPase. Bull. Japan. Soc. Sci. Fish. 43: 857-867 (in Japanese).
Kazak, M. 1987. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 15: 8125-8132.
Kohn, W.D., Monera, O.D., Kay, C.M. and Hodges, R.S. 1995. The effects of interhelical electrostatic repulsions between glutamic acid residues in controlling the dimerization and stability of twostranded alpha-helical coiled-coil. J. Biol. Chem. 270: 25495-5506.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685.
Lupas, A., Dyke, M.V. and Stock, J. 1991. Predicting coiled coils from protein sequences. Science 252: 1162-1164.
Lupas, A. 1996. Prediction and analysis of coiled-coil structures. Methods Enzymol. 266: 513-525.
McLachlan, A.D. and Karn, J. 1982. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature 299: 226-231.
Nakaya, T. Watabe, S. and Ooi, T. 1995. Differences in the thermal stability of acclimation temperature-associated types of carp myosin and its rod differential scanning calorimetory. Biochemistry 34: 3114-3120.
Nitta, T. and Itazawa, Y. 1980. Fish. In Suisan Seibutsu Tekisuionzu. Vol.1, pp. 7-29. Edited by Nitta, T. and Itazawa, Y. Japan Fisheries Resource Conservation Association, Tokyo (in Japanese).
Ogawa, M., Miyagi, R., Tamiya, T. and Tsuchiya,T. 1999. Comparison of the stability of fish light meromyosins by guanidine hydrochloride denaturetion. Comp. Biochem.Physiol. 122B: 439-446.
Ogawa, M., Tamiya, T. and Tsuchiya, T. 1994. Structural Changes of Carp Myosin during Heating. Fish. Sci. 60: 723-727.
Ogawa, M., Tamiya, T. and Tsuchiya, T. 1995. Thermal stability of α-helical structure of fishmyosin rods. Comp. Biochem. Physiol. 110B: 367-370.
Ogawa, M., Tamiya, T. and Tsuchiya, T. 1996. α-helical structure of fish actomyosin changes during storage. J. Agric. Food Chem. 44: 2944-2945.
Rodgers, M.E., Karr, T., Biedermann, K., Ueno, H. and Harrington, W.F. 1987. Thermal stability of myosin rod from various species. Biochemistry 26: 8703-8708.
Salamov, A.A. and Solovyev, V.V. 1995 Prediction of protein secondary sturcture by combining nearest-neighbor algorithms and multiply sequence alignments. J.Mol.Biol., 247: 11-15.
Sohn, R.L., Vikstrom, K.L., Strauss, M., Cohen, C., Szent-Gyorgyi, A.G. and Leinwand L.A. 1997. A29 residue region of the sarcomeric myosin rod is necessary for filamentformation. J. Mol. Biol. 266: 317-330.
Umemoto, S. 1966. Amodified method for estimation of fishmuscle protein by the biure method. Bull. Japan. Soc. Sci. Fish. 32: 427-435 (in Japanese).
Watabe, S., Hirayama, Y., Nakaya, M., Kakinuma, M., Kikuchi, K., Guo, X.-F., Kanoh, S., Chaen, S. and Ooi, T. 1997. Carp expresses fast skeletal myosin isoforms with altered motor functions and structural stabilities to compensate for changes in environmental temperature. J. Therm. Biol. 22: 375-390.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawabata, R., Kanzawa, N., Ogawa, M. et al. Determination of primary structure of amberjack myosin heavy chain and its relationship with structural stability of various fish myosin rods. Fish Physiology and Biochemistry 23, 283–294 (2000). https://doi.org/10.1023/A:1011176105285
Issue Date:
DOI: https://doi.org/10.1023/A:1011176105285