Abstract
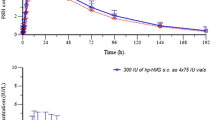
Human follicle stimulating hormone is a pituitary glycoprotein that is essential for the maintenance of ovarian follicle development and testicular spermatogenesis. Like other members of the glycoprotein hormone family, it contains a common a subunit and a hormone specificβ subunit. Each subunit contains two glycosylation sites. The specific structures of the oligosaccharides of human follicle stimulating hormone have been shown to influence both thein vitro andin vivo bioactivity. Since the carbohydrate structure of a protein reflects the glycosylation apparatus of the host cells in which the protein is expressed, we examined the isoform profiles,in vitro bioactivity and metabolic clearance of a preparation of purified recombinant human follicle stimulating hormone derived from a stable, transfected Sp2/0 myeloma cell line, and pituitary human follicle stimulating hormone. Isoelectric focussing and chromatofocussing studies of human follicle stimulating hormone preparations both showed a more basic isoform profile for the recombinant human follicle stimulating hormone compared to that of pituitary human follicle stimulating hormone. The recombinant human follicle stimulating hormone had a significantly higher radioreceptor activity compared to that of pituitary human follicle stimulating hormone, consistent with a greaterin vitro potency. Pharmacokinetic studies in rats indicated a similar terminal half life (124 min) to that of the pituitary human follicle stimulating hormone (119 min). Preliminary carbohydrate analysis showed recombinant human follicle stimulating hormone to contain high mannose and/or hybrid type, in addition to complex type carbohydrate chains, terminating with bothα2,3 andα2,6 linked sialic acids. These results demonstrate that recombinant human follicle stimulating hormone made in the Sp2/0 myeloma cells is sialylated, has a more basic isoform profile, and has a greaterin vitro biological potency compared to those of the pituitary human follicle stimulating hormone.
Similar content being viewed by others
Abbreviations
- LH:
-
Luteinising hormone
- FSH:
-
follicle stimulating hormone
- rhFSH:
-
recombinant human follicle stimulating hormone
- TSH:
-
thyroid stimulating hormone
- hCG:
-
human chorionic gonadotrophin
References
Bibila TA and Robinson DK (1995) In pursuit of the optimal fed-batch process for monoclonal antibody production. Biotechnol Prog 11: 1–13.
Bishop LA, Nguyen TV and Schofield PR (1995) Increased biological activity due to basic isoforms in recombinant human follicle stimulating hormone produced in a human cell line. Endocrinology 136: 2635–2640.
Bogdanove EM, Campbell GT, Blair ED, Mula ME, Miller AE and Grossman GH (1974) Gonadal pituitary feedback involves qualitative change: androgens alter the type of FSH secreted by the rat pituitary. Endocrinology 95: 219–228.
Briggs DW, Fisher JW and George WJ (1974) Hepatic clearance of intact and desialylated erythropoietin. Am J Physiol 227: 1385–1388.
Cerpa-Poljak A, Bishop LA, Hort Y, Chin CKH, DeKroon R, Mahler SM, Smith GM, Stuart MCJ and Schofield PR (1993) Isoelectric charge of recombinant human follicle stimulating hormone isoforms determines receptor affinity andin vitro bioactivity. Endocrinology 132: 351–356.
Chotigeat W, Wantanapokasin Y, Mahler SM and Gray PP (1995) Role of environmental conditions on the expression levels, glycoform pattern and levels of sialytransferase for hFSH produced by recombinant CHO cells. Cytotechnology 15: 217–221.
De Boer W and Mannerts B (1990) Recombinant follicle stimulating hormone II. Biochemical and biological characteristics. In: Crommelin DJA, Schellekens H (eds) From Clone to Clinic, Developments in Biotherapy. Kluwer, Dordrecht, vol. 1: 253–259.
Dorrington JH and Armstrong DT (1975) Follicle-stimulating hormone stimulatesβ-17 estradiol synthesis in cultured Sertoli cells. Proc Natl Acad. Sci USA 72: 2677–2681.
Flack MR, Bennet AP, Froehlich J, Anasti JN and Nisula BC (1994) Increased biological activity due to basic isoforms in recombinant human follicle stimulating hormone produced in a human cell line. J. Clin Endocrinol Metab 79: 756–760.
Gesundheit N, Magner JA, Chen T and Weintraub BD (1986) Differential sulphation and sialylation of secreted mouse thyrotropin subunits: regulation by TSH releasing hormone. Endocrinology 119:455–463.
Green ED and Baenziger JU (1988) Asparagine linked oligosaccharides on lutropin, follitropin and tyrotropin II. Distribution of sulphated and sialylated oligosaccharides on bovine, ovine and human pituitary glycoprotein hormones, J Biol Chem 263: 36–44.
Hard K, Mekking A, Damm JBL, Kamerling JP, de Boer W, Wijnands RA and Vliegenthart JFG (1990) Isolation and structure determination of the intact sialylated N-linked carbohydrate chains of recombinant human follitropin (hFSH) expressed in Chinese hamster ovary cells. Eur J Biochem 193: 262–271.
Harlin J, Khan SA and Diczfalusy E (1986) Molecular composition of luteinising hormone and follicle stimulating hormone in commercial gonadotropin preparations. Fertil Steril 46: 1055–1061.
Hori R, He YL, Shima T, Inui KI, Aoki S, Okumura K and Tanigawara Y (1992) Total body and hepatic clearance in rats of recombinant tissue-type plasminogen activator expressed in mouse C127 and Chinese hamster ovary cells. Drug Metab Dispos Biol Fate Chem 20: 541–546.
Keel BA and Grotjan HE (1989) Microheterogeneity of the Glycoprotein Hormones. In: Keel BA, Grotjan HE (eds) CRC Press, Boca Raton, 149–184.
Keene JL, Matzuk MM, Otani T, Fauser BCJM, Galway AR, Hsueh AJW and Boime I (1989) Expression of biologically active human follitropin in Chinese hamster ovary cells. J Biol Chem 246: 4769–4775.
Kentzer EJ, Buko A, Menon G and Sarin UK (1990) Carbohydrate composition and presence of a fucose-protein linkage in recombinant human pro-urokinase. Biochem Biophys Res Comm 171: 401–406.
Kornfeld S and Mellman I (1989) The biogenesis of lysosomes. Annu Rev Cell Biol 5: 483–525.
Lehrman MA and Hill RL (1986) The binding of fucose containing glycoprotein by hepatic lectins. Purification of a fucose binding lectin from rat liver. J Biol Chem 261: 7419–7425.
Mannerts B, de Leeuw R, van Ravenstein A, van Wezenbeek P, Schwurs A and Kloosterboer H (1991) Comparativein vitro andin vivo studies on the biological characterisation of recombinant human follicle-stimulating hormone. Endocrinology 129: 2623–2630.
Miller C, Ulloa-Aguirre A, Hyland L and Chappel S (1983) Pituitary follicle stimulating hormone heterogeneity: assesment of biological activities of each follicle stimulating hormone form. Fertil Steril 40: 242–247.
Mitchell CA, Beall JA, Wells JRE and Gray PP (1991) Growth and production kinetics of a murine myeloma cell line transfected with the human growth hormone gene. Cytotechnology 5: 223–231.
Parekh RB, Dwek RA, Rudd PM, Thomas JR and Rademacher TW (1989) N-glycosylation andin vitro enzymatic activity of human recombinant tissue plasminogen activator expressed in Chinese hamster ovary cells and a murine cell line. Biochemistry 28: 7670–7679.
Peckham WD and Knobil E (1976) The effects of ovariectomy, estrogen replacement and neuraminidase treatment upon the properties of the adenohyphophysical glycoprotein hormones of the rhesus monkey. Endocrinology 98: 1954–1060.
Pigny P, Berault A, Dewailly D and Boersma A (1992) Glycoprotein hormones, glycogylation and biological activity. Ann Biol Clin Paris 50(8): 557–564.
Rebois RV and Liss MT (1987) Antibody binding to theβ subunit of deglycosylated chorionic gonadotropin converts the antagonist to an agonist. J Biol Chem 262: 3891–3896.
Reichert Jr LE (1971) Electrophoretic properties of pituitary gonadotropins as studied by electrofocussing. Endocrinology 88: 1029–1044.
Reichert Jr LE and Bhalla VK (1994) Development of a radioligand tissue receptor assay of human follicle stimulating hormone. Endocrinology 94: 483–491.
Ritzen EM, Froysa B, Gustafson B, Westerhohn G and Diczfalusy E (1982) Improvedin vitro bioassay of follitropin. Hormone Research. 16: 42–48.
Ryan RJ, Keutman HT, Charlesworth MC, McCormick DJ, Milius RP, Calvo FO and Vutyavanich T (1987) Structure-function relationships of gonadotropins. Recent Prog Horm res 43: 383–429.
Sairam MR (1985) Protein glycosylation and receptor-ligand interactions. In: Conn PM (ed) The Receptors. Academic Press, New York, vol 2: 307–340.
Sherins RJ, Vaitukaitis JL and Charambach A (1973) Physical characterization of hFSH and its desialylation products by isoelectric focussing and electrophoresis in polyacrylamide gel. Endocrinology 92: 1135–1141.
Shulman M, Wilde CD and Kohler G (1978) A better cell line for making hybridomas secreting specific antibody. Nature 276: 269–270.
Stanton PG, Robertson DM, Burgon PG, Schmauk-White B and Hearn MTW (1992) Isolation and physicochemical characterization of human follicle-stimulating hormone isoforms. Endocrinology 130:2820–2831.
Ulloa-Aguirre A, Miller C, Hyland L and Chappel SC (1984) Production of all FSH isohormones from a purified FSH preparation by neuraminidase digestion. Biol Reprod 30: 382–387.
Ulloa-Aguirre A, Cravioto A, Damian-Matsumura P, Jimenez M, Zambrano E and Diaz-Sanchez V (1992) Biological characterization of the naturally occuring analogues of intrapituitary human follicle stimulating hormone. Human reprod 7: 23–30.
Vaitukaitis JL and Ross GT (1971) Altered biologic and immunologic activities of progressively desialylated human urinary FSH. J Clin Endocrinol Metab 33: 308–311.
Van Damme MP, Robertson DM, Marana R, Ritzen EM and Diczfalusy E (1979) A sensitive and specificin vitro bioassay method for the measurement of follicle stimulating hormone activity. Acta Endocrinal (Copenh) 91: 224–237.
Van Wezenbeek P, Draaier J, Van Meel F and Olijve W (1990) Recombinant follicle stimulating hormone I. Construction, selection and characterisation of a cell line. In: Crommelin DJA, Schellekens H (eds) From Clone to Clinic, developments in Biotherapy. Kluwer, Dordrecht, vol 1: 245–251.
Weidle UH and Buckel P (1987) Establishment of stable mouse myeloma cells constitutively secreting human tissue-type plasminogen activator. Gene 57: 131–141.
Wide L (1982) Male and female forms of human follicle stimulating hormone in serum. J Clin Endocrinol Metab 55: 682–688.
Wide L and Hobson B (1986) Influence of the assay method used on the selection of the most active forms of FSH from the human pituitary. Acta Endocrinol (Copenh) 113: 17–22.
Zaidi AA, Roberson DM and Diczfalusy E (1981) Studies on the biological and immunological properties of human follitropin: profiles of two international reference preparations and of an aqueous extract of pituitary glands after electrofocussing. Acta Endocrinol (Copenh) 97: 157–165.
Zaida AA, Frosa B and Diczfalusy E (1982) Biological and immunological properties of different molecular species of human follicle stimulating hormone: electrofocussing profiles of eight highly purified preparations. J Endocrinol 92: 195–204.
Zambrano E, Olivares A, Mendez JP, Guerro L, Diaz-Cueto L, Veldhuiss JD and Ulloa-Aguirre A (1995) Dynamics of basal and gonadotropin-releasing hormone-releasable serum folliclestimulating hormone charge isoform distribution throughout the human menstrual cycle. J Clin Endocrinol Metab 80: 1647–1656.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chin, C.K., Schofield, P.R., Robertson, D.M. et al. Biological activity and metabolic clearance of recombinant human follicle stimulating hormone produced in Sp2/0 myeloma cells. Cytotechnology 21, 171–182 (1996). https://doi.org/10.1007/BF02215667
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02215667