Abstract
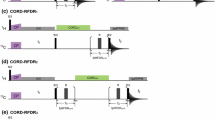
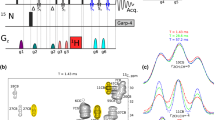
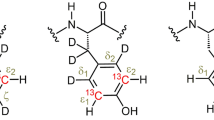
Constant-time 3D heteronuclear relayed E.COSY [Schmidt et al. (1996) J. Biomol. NMR, 7, 142–152], as based on generic 2D small-flip-angle HMQC-COSY [Schmidt et al. (1995) J. Biomol. NMR, 6, 95–105], has been modified to allow for quantitative determination of heteronuclear three-bond 3 J(Hα,Cγ) couplings. The method is applicable to amino acid spin topologies with carbons in the γ position which lack attached protons, i.e. to asparagine, aspartate, and aromatic residues in uniformly 13C-enriched proteins. The pulse sequence critically exploits heteronuclear triple-quantum coherence (HTQC) of CH2 moieties involving geminal Hβ proton pairs, taking advantage of improved multiple-quantum relaxation properties, at the same time avoiding scalar couplings between those spins involved in multiple-quantum coherence, thus yielding E.COSY-type multiplets with a splitting structure that is simpler than with the original scheme. Numerical least-squares 2D line-shape simulation is used to extract 3 J(Hα,Cγ) coupling constants which are of relevance to side-chain χ1 dihedral-angle conformations in polypeptides. Methods are demonstrated with recombinant 15N,13C-enriched ribonuclease T1 and Desulfovibrio vulgaris flavodoxin with bound oxidized FMN.
Similar content being viewed by others
References
Aue, W.P., Bartholdi, E. and Ernst, R.R. (1976) J. Chem. Phys., 64 2229–2246.
Bax, A., Vuister, G.W., Grzesiek, S., Delaglio, F., Wang, A.C., Tschudin, R. and Zhu, G. (1994) Methods Enzymol., 239, 79–105.
Blümel, M., Schmidt, J.M., Löhr, F. and Rüterjans, H. (1998) Eur.Biophys. J., 27, 321–334.
DeMarco, A., Llinás, M. and Wüthrich, K. (1978) Biopolymers, 17, 617–636.
DeMarco, A. and Llinás, M. (1979) Biochemistry, 18, 3846–3854.
Eggenberger, U., Schmidt, P., Sattler, M., Glaser, S.J. and Griesinger, C. (1992) J. Magn. Reson., 100, 604–610.
Emsley, L. and Bodenhausen, G. (1990) Chem. Phys. Lett., 165, 469–476.
Ernst, R.R., Bodenhausen, G. and Wokaun, A. (1987) Principles of Nuclear Magnetic Resonance in One and Two Dimensions, Clarendon Press, Oxford, U.K.
Griesinger, C., Sørensen, O.W. and Ernst, R.R. (1986) J. Chem. Phys., 85, 6837–6852.
Griffey, R.H. and Redfield, A.G. (1987) Quart. Rev. Biophys., 19, 51–82.
Grzesiek, S. and Bax, A. (1993) J. Biomol. NMR, 3, 185–204.
Grzesiek, S. and Bax, A. (1995) J. Biomol. NMR, 6, 335–339.
Gschwind, R.M., Gemmecker, G. and Kessler, H. (1998) J. Biomol. NMR, 11, 191–198.
Karimi-Nejad, Y. (1994) Thesis, University of Cologne, Germany.
Logan, T.M., Olejniczak, E.T., Xu, R.X. and Fesik, S.W. (1992) FEBS Lett., 314, 413–418.
Löhr, F., Blümel, M., Schmidt, J.M. and Rüterjans, H. (1997) J. Biomol. NMR, 10, 107–118.
Löhr, F. and Rüterjans, H. (1995) J. Biomol. NMR, 5, 25–36.
Löhr, F. and Rüterjans, H. (1999) J. Biomol. NMR, 13, 263–274.
Marino, J.P., Diener, J.L., Moore, P.B. and Griesinger, C. (1997) J. Am. Chem. Soc., 85, 2870–2871.
Marion, D., Ikura, M., Tschudin, R. and Bax, A. (1989) J. Magn. Reson., 85, 393–399.
Martinez-Oyanedel, J., Choe, H.W., Heinemann U. and Saenger, W. (1991) J. Mol. Biol., 222, 335–352.
Mattiello, D.L., Warren, W.S., Mueller, L. and Farmer II, B.T. (1996) J. Am. Chem. Soc., 118, 3253–3261.
Norwood, T.J. (1992) Prog. NMR Spectrosc., 24, 295–375.
Piantini, U., Sørensen, O.W. and Ernst, R.R. (1982) J. Am. Chem. Soc., 104, 6800–6801.
Schmidt, J.M. (1997) J. Magn. Reson., 124, 298–309.
Schmidt, J.M., Blümel, M., Löhr, F. and Rüterjans, H. (1999) J. Biomol. NMR, 14, 1–12.
Schmidt, J.M., Ernst, R.R., Aimoto, S. and Kainosho, M. (1995) J. Biomol. NMR, 6, 95–105.
Schmidt, J.M., Löhr, F. and Rüterjans, H. (1996) J. Biomol. NMR, 7, 142–152.
Schmidt, J.M. and Rüterjans, H. (1990) J. Am. Chem. Soc., 112, 1279–1280.
Seip, S., Balbach, J. and Kessler, H. (1992) J. Magn. Reson., 100, 406–410.
Shaka, A.J., Barker, P.B. and Freeman, R. (1985) J. Magn. Reson., 64, 547–552.
Sklenár, V., Dieckmann, T., Butcher, S.E. and Feigon, J. (1998) J. Magn. Reson., 130, 119–124.
Smallcombe, S.H., Patt, S.L. and Keifer, P.A. (1995) J. Magn. Reson., A117, 295–303.
Swapna, G.V.T., Rios, C.B., Shang, Z. and Montelione, G.T. (1997) J. Biomol. NMR, 9, 105–111.
Tessari, M., Gentile, L.N., Taylor, S.J., Shalloway, D.I., Nicholson, L.K. and Vuister, G.W. (1997) Biochemistry, 36, 14561–14571.
Vuister, G.W. and Bax, A. (1993) J. Magn. Reson., B102, 228–231.
Wasylishen, R. and Schaefer, T. (1972) Can. J. Chem., 50, 2710–2712.
Watt, W., Tulinsky, A., Swenson, R.P. and Watenpaugh, K.D. (1991) J. Mol. Biol., 218, 195–208.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Löhr, F., Pérez, C., Köhler, R. et al. Heteronuclear relayed E.COSY revisited: Determination of 3J(Hα,Cγ) couplings in Asx and aromatic residues in proteins. J Biomol NMR 18, 13–22 (2000). https://doi.org/10.1023/A:1008385202639
Issue Date:
DOI: https://doi.org/10.1023/A:1008385202639