Abstract
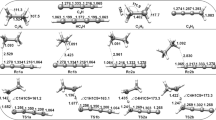
Reactions of the methoxide ion with substituted halocyclopropanes, which result in E2 elimination, have been studied by the semiempirical quantum-chemical AM1 method. The transition states corresponding totrans andcis routes have been localized. The energetic predominance of thetrans route over thecis route is reduced by 2.6 kcal mol−1 on going from 1-chloropropane to chlorocyclopropane because of the features of cyclopropane geometry. It has been demonstrated that, in the gas phase,cis elimination may predominate overtrans elimination for a particular stereoisomer of 2-cyano-2-methyl-1-halocyclopropanes due to weakening of orbital interactions and Coulomb repulsion between the cyano group and the MeO− anion in thetrans E2 transition state.
Similar content being viewed by others
References
Z. Shi and R. J. Boyd,J. Am. Chem. Soc., 1990,112, 6789 and references therein.
K. Fukui, H. Hao, and H. Fujimoto,Bull. Chem. Soc. Jpn., 1969,42, 348.
H. Fujimoto, S. Yamah, and K. Fukui,Bull. Chem. Soc. Jpn., 1971,44, 971.
R. D. Bach, R. C. Badger, and T. J. Lang,J. Am. Chem. Soc., 1979,94, 3718.
T. Minato and S. Yamabe,J. Am. Chem. Soc., 1985,107, 4621.
M. J. Dewar and Y. C. Yuan,J. Am. Chem. Soc., 1990,112, 2088.
M. J. Dewar and Y. C. Yuan,J. Am. Chem. Soc., 1990,112, 2095
S. Gronert,J. Am. Chem. Soc., 1991,113, 6041.
S. Gronert,J. Am. Chem. Soc., 1992,114, 2349.
F. M. Bickelhaupt, E. J. Baerends, N. M. M. Nibbering, and T. Ziegler,J. Am. Chem. Soc., 1993,113, 9160.
N. D. Epiotis, W. R. Cherry, S. Shaik, R. Yates, and F. Bernardi,Structural Theory of Organic Chemistry. Top. Curr. Chem., 1977,70, 250.
I. G. Bolesov, V. V. Plemenkov, inSovremennye problemy organicheskoi khimii [Modern Problems of Organic Chemistry], Izd. LGU, Leningrad, 1987, No. 9, 137–161 (in Russian).
K. S. Brown and W. H. Ir. Saunders,J. Am. Chem. Soc., 1970,92, 4292.
N. I. Yakushkina and I. G. Bolesov,Zh. Org. Khim., 1980,16, 1335 [J. Org. Chem. USSR, 1980,16 (Engl. Transl.)].
M. J. S. Dewar, E. G. Zoebisch, E. F. Healy, and J. J. P. Stewart,J. Am. Chem. Soc., 1985,107, 3902.
M. J. S. Dewar and K. M. Dieter,J. Am. Chem. Soc., 1986,108, 8075.
M. J. S. Dewar, E. G. Zoebisch, E. F. Healy, and J. J. P. Stewart,AMPAC(IBM), QCPE, No. 527, 1987.
M. J. S. Dewar and S. J. Kischner,J. Am. Chem. Soc., 1971,93, 4290.
J. W. McIver and A. Komornicki,J. Am. Chem. Soc., 1972.94, 2625.
T. Su and M. T. Bowers, inGas Phase Ion Chemistry, Academic Press, New York, 1979, 1.
Author information
Authors and Affiliations
Additional information
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 4, pp. 620–623, April, 1995.
Rights and permissions
About this article
Cite this article
Ermolaeva, L.V., Appolonova, S.A., Plemenkov, V.V. et al. Theoretical study of bimolecular elimination (E2) reactions. Possibility ofsyn E2 elimination in the series of 2-R-2-R'-1-halocyclopropanes. Russ Chem Bull 44, 599–602 (1995). https://doi.org/10.1007/BF00698486
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00698486