Abstract
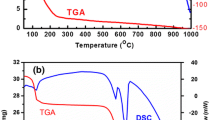
Sol-gel synthesis of nano-sized BaTiO3, BaZrO3 and BaTi0.5Zr0.5O3 ceramics using alkoxide and semi-alkoxide routes has been investigated and the pervoskites obtained have been compared with respect to crystallisation temperature, crystallite size and compositional purity. Heterometal alkoxides containing two (for BaTiO3 and BaZrO3) and three (for BaTi0.5Zr0.5O3) different metals were used as single-source precursors in the alkoxide route while semi-alkoxide synthesis was performed by reacting barium hydroxide or acetate with Ti and/or Zr alkoxides. Semi-alkoxide synthesis also produces stoichiometric and phase-pure oxides, however, at temperatures higher than 1000°C. At temperatures below 1000°C, BaCO3 and small amounts of other undesired phases (e.g., BaTi2O4) were present in the oxides derived from semi-alkoxide synthesis. Thermal behaviour, studied by TGA/DTA measurements, shows that thermal decomposition occurs in three major steps and depends on the educt composition and the synthesis route. Among alkoxide derived powders, crystalline BaTi0.5Zr0.5O3 phase is formed at 400°C while complete crystallisation of BaMO3 ceramics occurs around 600°C. The cubic to tetragonal phase transition for BaTiO3 is clearly observed at relatively low-temperature of 800°C. The stoichiometry and phase homogeneity of the obtained powders were demonstrated by energy dispersive X-ray analysis and powder diffractometry. The averaged crystallite size of the obtained nano-ceramics was evaluated using the FormFit programme. SEM and TEM observations revealed a high microstructural uniformity.
Similar content being viewed by others
REFERENCES
A.J. Moulson and J.M. Herbert, Electroceramics: Materials, Properties and Applications (Chapman and Hall, London, 1986).
G. Goodman, in Ceramic Materials for Electronics, edited by R.C. Buchanan (Marcel-Dekker, New York, 1986).
E. Wu, K.C. Chen, and J.D. Mackenzie, in Better Ceramics Through Chemistry, edited by C.J. Brinker, D.E. Clark, and D.R. Ulrich (North Holland, Amsterdam, 1984).
R.E. Riman, in High-performance Ceramics, edited by R. Pugh and L. Bergstroem (Marcel-Dekker, New York, 1993).
R.C. Mehrotra, Chemtracts 2, 338 (1990).
C.D. Chandler, C. Roger, and M.J. Hampden-Smith, Chem. Rev. 93, 1205 (1993).
L.L. Hench and J.K. West, Chem. Rev. 90, 33 (1990).
K. Uchino, E. Sadanaga, and T. Hirose, J. Am. Ceram. Soc. 72, 1555 (1989).
P.P. Phule and S.H. Risbud, J. Mater. Sci. 25, 1169 (1990).
H.P. Beck, F. Mueller, R. Haberkorn, and D. Wilhelm, Nanostructured Materials 6, 659 (1995).
B. Gissibl, D. Wilhelm, R. Wuerschum, H. Herrig, F. Mueller, M. Kelsch, K. Reimann, F. Phillipp, H.P. Beck, R. Hempelmann, and H.E. Schaefer, Nanostructured Materials 9, 619 (1997).
K.K. Deb, M.D. Hill, and J.F. Kelly, J. Mater. Res. 7, 3296 (1992).
T.R. Armstrong, L.E. Morgens, A.K. Maurice, and R.C. Buchanan, J. Am. Ceram. Soc. 72, 605 (1989).
D.M. Tahan, A. Safari, and L.C. Klein, J. Am. Ceram. Soc. 79, 1593 (1996).
N. Kamehara, M. Tsukada, J.S. Cross, and K. Kurihara, J. Ceram. Soc. Jpn. 105, 801 (1997).
G. Pfaff, J. Mater. Chem. 2, 591 (1992).
P. Nanni, M. Leoni, V. Buscaglia, and G. Aliprandi, J. Europ. Ceram. Soc. 14, 85 (1990).
C. Lemoine, B. Gilbert, B. Michaux, J.P. Pirard, and A.J. Lecloux, J. Non-Cryst Solids 1, 175 (1994).
M. Veith, S. Mathur, and C. Mathur, Polyhedron 17, 1005 (1998).
R.C. Mehrotra, A. Singh, and S. Sogani, Chem. Rev. 94, 1643 (1994).
R.C. Mehrotra, J. Non-Cryst. Solids 100, 1 (1988).
Y. Suyama, and M. Nagawara, J. Am. Ceram. Soc. 77, 603 (1994).
E.P. Turevskaya, D.V. Berdyev, and N. Ya. Turova, J. Sol-Gel Sci. Tech. 8, 111 (1997).
L.G. Hubert-Pfalzgraf, S. Daniele, J.M. Decans, and J. Vaissermann, J. Sol-Gel Sci. Tech. 8, 49 (1997).
R.E. Rocheleau, Z. Zhang, J.W. Gilje, and J.A. Meese-Marktscheffel, Chem. Mater. 6, 1615 (1994).
K.B. Pflanz, R. Riedel, and H. Schmiel, Adv. Mater. 4, 662 (1992).
D.J. Eichorst and D.A. Payne, Mater. Res. Soc. Symp. Proc. 271, 331 (1992).
M. Veith and S. Kneip, J. Mater. Sci. Lett. 13, 335 (1994).
M. Veith, S. Faber, R. Hempelman, S. Janssen, J. Prewo, and H. Eckerlebe, J. Mater. Sci. 31, 2009 (1996).
M. Veith, S. Kneip, A. Jungmann, and S. Hüfner, Z. Anorg. Allg. Chem. 623, 1507 (1997).
M. Veith, A. Altherr, N. Lecerf, S. Mathur, K. Valtchev, and E. Fritscher, Nanostructured Materials 12, 191 (1999).
D.C. Bradley, R.C. Mehrotra, and D.P. Gaur, Metal Alkoxides (Academic Press, London, 1978).
R. Haberkorn, FormFit—Programme for Particle Size Distribution From XRD Scans (University of Saarland, Saarbruecken, Germany, 1997).
M. Veith, S. Mathur, V. Huch, and T. Decker, Eur. J. Inorg. Chem. 1327 (1998).
M. Veith and S. Mathur, manuscript in preparation.
V.W. Day, T.A. Eberspacher, W.G. Klemperer, and C.W. Park, J. Am. Chem. Soc. 115, 8469 (1993).
V.W. Day, T.A. Eberspacher, W.G. Klemperer, C.W. Park, and F.S. Rosenberg, J. Am. Chem. Soc. 113, 8190 (1991).
V.W. Day, T.A. Eberspacher, W.G. Klemperer, C.W. Park, and F.S. Rosenberg, in Chemical Processing of Advanced Materials, edited by L.L. Hench and J.K. West (Wiley, New York, 1992).
JCPDS Powder Diffraction File, File Card Nos. [5–626], [31–174] and [6–399] Joint Committee for Powder Diffraction Standards (1990).
A.R. West, Solid State Chemistry VCH (1992).
H.P. Beck, R. Haberkorn, and W. Eiser, manuscript in preparation.
R.A. Nyquist, C.L. Putzy, and M.A. Leugers, The Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts (Academic Press, 1997).
M. Veith, A. Altherr, and H. Wolfanger, Adv. Materials 5, 87 (1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Veith, M., Mathur, S., Lecerf, N. et al. Sol-Gel Synthesis of Nano-Scaled BaTiO3, BaZrO3 and BaTi0.5Zr0.5O3 Oxides via Single-Source Alkoxide Precursors and Semi-Alkoxide Routes. Journal of Sol-Gel Science and Technology 17, 145–158 (2000). https://doi.org/10.1023/A:1008795419020
Issue Date:
DOI: https://doi.org/10.1023/A:1008795419020