Summary
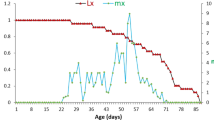
Our paper addresses field survivorship of first instar monarch butterfly larvae (Danaus plexippus L., Lep.: Danainae) in relation to the dual cardenolide and latex chemical defenses of the sand hill milkweed plant,Asclepias humistrata (Asclepiadaceae) growing naturally in north central Florida. Survival of first instar larvae in the field was 11.5% in the first experiment (15–20 April 1990), and dropped to 3.4% in the second experiment (20–30 April). About 30% of the larvae were found glued to the leaf surface by the milkweed latex. Predator exclusion of non-flying inverte-brates by applying “tanglefoot” to the plant stems suggested that the balance of the mortality was due to volant inverte-brates, or to falling and/or moving off the plants. Regression analyses to isolate some of the other variables affecting survivorship indicated that first instar mortality was correlated with (1) increasing cardiac glycoside concentration of the leaves, (2) increasing age of the plants, and (3) the temporal increase in concentration of cardiac glycosides in the leaves. The study also provided confirmatory data of previous studies that wild monarch females tend to oviposit onA. humistrata plants containing intermediate concentrations of cardiac glycosides. Cardiac glycoside concentration in the leaves was not correlated with that in the latex. The concentration of cardenolide in the latex is extremely high, constituting an average of 1.2 and 9.5% of the mass of the wet and dry latex, respectively. The data suggest that an increase in water content of the latex is compensated for by an influx of cardenolide with the result that the cardenolide concentration remains constant in the latex systems of plants that are growing naturally. We also observed first instar larvae taking their first bite of milkweed leaves in the field. In addition to confirming other workers findings that monarch larvae possess elaborate “sabotaging” behaviour of the milkweed's latex system, we discovered that several larvae on their first bite involuntarily imbided a small globule of latex and instantly became cataleptic. This catalepsis, lasting up to 10 min, may have been in response to the high concentration of cardenolide present in the latex ofA. humistrata, more than 10 times that in the leaves. The results of the present study suggest that more attention should be directed to plant chemical defenses upon initial attack by first instar insect larvae, rather than attempting correlations of plant chemistry with older larvae that have already passed the early instar gauntlet. The first bite of neonate insects may be the most critical moment for coping with the chemical defenses of many plants and may play a much more important role in the evolution of insect herbivory than has previously been recognized.
Similar content being viewed by others
References
Ackery PR, Vane-Wright RI (1984) Milkweed Butterflies: Their Cladistics and Biology. Ithaca: Cornell University Press
Aide TM, Londono EC (1989) The effects of rapid leaf expansion on the growth and survivorship of a lepidopteran herbivore. Oikos 55:66–70
Ambatali FA (1990) Influence of host plants on the development, feeding behaviour and parasitism ofEuploea core corinna WS Macleay (Lepidoptera: Nymphalidae). Master of Agric Studies Thesis, University of Queensland
Blaser HW (1945) Anatomy ofCryptostegia grandiflora with special reference to the latex system. Am J Bot 32:135–141
Borkin SS (1982) Notes on shifting distribution patterns and survival of immatureDanaus plexippus (Lepidoptera: Danaidae) on the food plantAsclepias syriaca. Great Lakes Entomol 15:199–206
Brewer J (1977) Short lived phenomena. News Lepid Soc 4:7
Brower LP (1969) Ecological chemistry. Sci Amer 200 (2):22–29
Brower LP (1984) Chemical defence in butterflies. Symp R Entomol Soc Lond 11:109–134 (= Vane-Wright RI, Ackery PR (eds) The Biology of Butterflies. London: Academic Press)
Brower LP, Brower JV (1964) Birds, butterflies, and plant poisons: a study in ecological chemistry. Zoologica 49:137–159
Brower LP, Fink LS (1985) A natural toxic defence system in butterflies versus birds. Ann NY Acad Sci 443:171–188
Brower LP, Brower JVZ, Corvino JM (1967) Plant poisons in a terrestrial food chain. Proc Natl Acad Sci USA 57:893–898
Brower LP, Ryerson WN, Coppinger LL, Glazier SC (1968) Ecological chemistry and the palatability spectrum. Science 161:1349–1351
Brower LP, McEvoy PB, Williamson KL, Flannery MA (1972) Variation in cardiac glycoside content of monarch butterflies from natural populations in eastern north America. Science 177:426–429
Brower LP, Edmunds M, Moffitt CM (1975) Cardenolide contents and palatability of a population ofDanaus chrysippus butterflies from West Africa. J Entomol 49:183–196
Brower LP, Nelson CJ, Seiber JN, Fink LS, Bond C (1988) Exaptation as an alternative to coevolution in the cardenolide-based chemical defence of monarch butterflies (Danaus plexippus L.) against avian predators. Pp 447–475in Spencer KC (ed.) Chemical Mediation of Coevolution. New York: Academic Press
Chapman RF, Bernays EA (1989) Insect behaviour at the leaf surface and learning as aspects of host plant selection. Experientia 45:215–222
Cohen JA (1983) Chemical interactions among milkweed plants (Asclepi-adaceae) and lepidopteran herbivores. PhD Thesis, University of Florida, Gainesville
Cohen JA, Brower LP (1982) Oviposition and larval success of wild monarch butterflies (Lepidoptera: Danaidae) in relation to host plant size and cardenolide concentration. J Kansas Entomol Soc 55:343–348
Compton SG (1987)Aganais speciosa andDanaus chrysippus (Lepidoptera) sabotage the latex defenses of their host plants. Ecol Entomol 12:115–118
Compton SG (1989) Sabotage of latex defenses by caterpillars feeding on fig trees. S Afr J Sci 85:605–606
Dempster JP (1983) The natural control of populations of butterflies and moths. Biol Rev 58:461–481
Dillon PM, Lowrie S, McKey D (1983) Disarming the “evil woman”: petiole constriction by a sphingid larva circumvents mechanical defenses of its hostplant,Cnidoscolus urens (Euphorbiaceae). Biotropica 15:112–116
Dixon CA, Erikson JM, Kellett DN, Rothschild M (1978) Some adaptations betweenDanaus plexippus and its food plant, with notes onDanaus chrysippus andEuploea core (Insecta: Lepidoptera). J Zool Lond 185:437–467
Dussourd DE (1986) Adaptations of insect herbivores to plant defences. PhD Thesis, Cornell University, Ithaca NY
Dussourd DE (1990) The vein drain; or how insects outsmart plants. Nat Hist 90:44–49
Dussourd DE, Eisner T (1987) Vein-cutting behaviour: insect counterploy to the latex defence of plants. Science 237:898–901
Edwards PJ, Wratten SD (1983) Wound induced defences in plants and their consequences for patterns of insect grazing. Oecologia 59:88–93
Erickson JM (1973) The utilization of variousAsclepias species by larvae of the monarch butterfly,Danaus plexippus. Psyche 80:230–244
Fisk J (1980) Effects of HCN, phenolic acids and related compounds inSorghum bicolor on the feeding behaviour of the planthopperPeregrinus maidis. Entomol exp appl 27:211–222
Fox LR, Macauley BJ (1977) Insect grazing onEucalyptus in response to variation in leaf tannins and nitrogen. Oecologia 29:145–162
Harborne JB (1988) Introduction to Ecological Biochemistry. 3rd ed. New York: Academic Press
Hassel MP (1985) Insect natural enemies as regulating factors. J Anim Ecol 54:323–334
Hutchins RFN, Sutherland ORW, Gnanasunderam C, Greenfield WJ, Williams EM, Wright HJ (1984) Toxicity of nitro compounds fromLotus pedunculatus to grass grub(Costelytra zealandica) (Coleoptera: Scarabeidae). J Chem Ecol 10:81–93
Isman MB, Duffey SS (1982) Toxicity of tomato phenolic compounds to the fruitworm,Heliothis zea. Entomol exp appl 31:370–376
James DG (1981) Studies on a winter breeding population ofDanaus plexippus (L.) (Lepidoptera: Nymphalidae) at Spencer, New South Wales. Gen Appl Entomol 13:47–53
Jungreis AM, Vaughan GL (1977) Insensitivity of lepidopteran tissue to ouabain: absence of ouabain binding and Na+, K+ ATPases in larval and adult midgut. J Insect Physiol 23:503–509
Kyi A, Zalucki MP, Titmarsh IJ (1991) Factors affecting the survival of the early stages ofHeliothis armigera (Huebner) (Lepidoptera: Noctuidae). Bull Entomol Res 81:263–271
Louda SM, Rodman JE (1983) Concentration of glucosinolates in relation to habitat and insect herbivory for the native cruciferCardamine cordifolia. Biochem System Ecol 11:199–207
Lucansky TW, Cloug KT (1986) Comparative anatomy and morphology ofAsclepias perennis andAsclepias tuberosa subspeciesrolfsii. Bot Gaz 147:290–301
Malcolm SB, Brower LP (1986) Selective oviposition by monarch butterflies (Danaus plexippus L.) in a mixed stand ofAsclepias curassavica L. andA. incarnata L. in south Florida. J Lep Soc 40:255–263
Malcolm SB, Brower LP (1989) Evolutionary and ecological implications of cardenolide sequestration in the monarch butterfly. Experientia 45:284–295
Malcolm SB, Zalucki MP (eds) (1992) Biology and Conservation of the Monarch Butterfly. Los Angeles: Natural History Museum of Los Angeles County
Malcolm SB, Cockrell BJ, Brower LP (1987) Monarch butterfly voltinism: effects of temperature constraints at different latitudes. Oikos 49:77–82
Malcolm SB, Cockrell BJ, Brower LP (1989) Cardenolide fingerprint of monarch butterflies reared on common milkweed,Asclepias syriaca L. J Chem Ecol 15:819–853
Mathavan S, Bhaskaran R (1975) Food selection and utilisation in a danaid butterfly. Oecologia 8:55–62
Miles PW, Aspinall D, Rosenberg L (1982) Performance of the cabbage aphid,Brevicoryne brassicae (L.), on water-stressed rape plants, in relation to changes in their chemical composition. Aust J Zool 30:337–345
Morrow PA, Fox LR (1980) Effects of variation inEucalyptus essential oil yields on insect growth and grazing damage. Oecologia 42:209–219
Nelson CJ (1992) Sequestration and storage of cardenolides and cardenolide glycosides byDanaus plexippus L. andD. chrysippus petilia (Stoff) when reared onAsclepias fruticosa L.; a review of some factors influencing sequestration process. In pressin Malcolm SB, Zalucki MP (eds) Biology and Conservation of the Monarch Butterfly. Los Angeles: Natural History Museum of Los Angeles County
Nelson CJ, Seiber JN, Brower LP (1981) Seasonal and intraplant variation of cardenolide content in the California milkweed,Asclepias eriocarpa, and implications for plant defence. J Chem Eco 7:981–1010
Nielsen PE, Nishimura H, Otvos JW, Calvin M (1977) Plant crops as a source of fuel and hydrocarbon-like materials. Science 198:942–944
Nishio S (1980) The fates and adaptive significance of cardenolides sequestered by larvae ofDanaus plexippus (L.) andCycnia inoptinatus (Hy. Edwards). PhD Thesis, University of Georgia
Orrell J (1970) The baby is a cannibal. Wildlife 7:44–47
Oyeyele S, Zalucki MP (1990) Cardiac glycosides and oviposition byDanaus plexippus onAsclepias fruticosa in south-east Queensland (Australia), with notes on the effects of plant nitrogen content. Ecol Entomol 15:177–185
Rawlins JE, Lederhouse RC (1981) Developmental influences of thermal behaviour on monarch caterpillars(Danaus plexippus): an adaptation for migration (Lepidoptera: Nymphalidae: Danainae). J Kansas Entomol Soc 54:387–408
Rothschild M (1977) The cat-like caterpillar. News Lepid Soc 6:9
Scriber JM (1981) Sequential diets, metabolic costs, and growth ofSpodoptera eridiana (Lepidoptera: Noctuidae) feeding on dill, lima beans, and cabbage. Oecologia 51:175–180
Seber GAF (1982) The Estimation of Animal Abundance. London: Griffin & Co
Seiber JN, Tuskes TM, Brower LP, Nelson CN (1980) Pharmacodynamics of some milkweed cardenolides fed to larvae of the monarch butterfly (Danaus plexippus L.). J Chem Ecol 6:321–339
Seiber JN, Nelson CJ, Lee SM (1982) Cardenolides in the latex and leaves of sevenAsclepias species andCalotropis procera. Phytochemistry 21:2343–2348
Singer MC (1984) Butterfly-hostplant relationships: host quality, adult choice and larval success. Symp R Entomol Soc Lond 11:81–88 (= Vane-Wright RI, Ackery PR (eds) The Biology of Butterflies. London: Academic Press)
Slansky F Jr, Feeny PP (1977) Maximization of the rate of nitrogen accumulation by larvae of the cabbage butterfly on wild and cultivated food plants. Ecol Monogr 47:209–228
Todd GW, Getahum A, Cress DC (1971) Resistance in barley to the greenbug,Schizaphis graminum. 1. Toxicity of phenolic and flavonoid compounds and related substances. Ann Entomol Soc Am 64:718–722
Van Emon JV, Seiber JN (1985) Chemical constituents and energy content of two milkweeds,Asclepias speciosa andA. curassavica. Econ Bot 39:47–55
Van Hook T, Zalucki MP (1991) Oviposition byDanaus plexippus onAsclepias viridis in northern Florida. J Lep Soc 45:215–221
Vaughan GL, Jungreis AM (1977) Insensitivity of lepidopteran tissue to oabian: physiological mechanisms for protection from cardiac glycosides. J Insect Physiol 23:585–589
Wilson KJ, Mahlberg PG (1980) Ultrastructure of developing and mature nonarticulated laticifers in the milkweed,Asclepias syriaca L. (Asclepiadaceae). Am J Bot 67:1160–1170
Zalucki MP, Kitching RL (1982a) Dynamics of oviposition inDanaus plexippus (Insecta: Lepidoptera) on milkweed,Asclepias spp. J Zool 198:103–116
Zalucki MP, Kitching RL (1982b) Temporal and spatial variation of mortality in field populations ofDanaus plexippus L. andD. chrysippus L. larvae (Lepidoptera: Nymphalidae). Oecologia 53:201–207
Zalucki MP, Oyeyele S, Vowles P (1989) Selective oviposition byDanaus plexippus L. (Lepidoptera: Nymphalidae) in a mixed stand ofAsclepias fruticosa andA. curassavica in southeast Queensland. J Aust Entomol Soc 28:141–146
Zalucki MP, Brower LP, Malcolm SB (1990) Oviposition byDanaus plexippus in relation to cardenolide content of threeAsclepias species in the southeastern U.S.A. Ecol Entomol 15:231–240
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zalucki, M.P., Brower, L.P. Survival of first instar larvae ofDanaus plexippus (Lepidoptera: Danainae) in relation to cardiac glycoside and latex content ofAsclepias humistrata (Asclepiadaceae). Chemoecology 3, 81–93 (1992). https://doi.org/10.1007/BF01245886
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01245886