Abstract
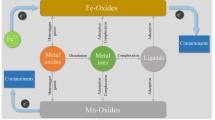
The kinetics of conversion of iron(III) (hydr)oxides to ferrous iron mediated by fulvic acid have been investigated in order to improve the understanding of the redox cycling of iron at the oxic-anoxic boundary in natural waters. Under the conditions similar to natural waters, fulvic acid is able to reduce the iron(III) (hydr)oxide. The kinetics of the reaction depend on the reactivity of iron(III) (hydr)oxides and the reducing power of the fulvic acid. The rate of reaction is 60 nm/h obtained under following conditions: total concentration of Fe(III) 1.0 × 10−4 M, pH 7.5, fulvic acid 5 mg/L. The rate is considered as a net result of reduction and oxidation in the > FeIII-OH/Fe(II) “wheel” coupled with fulvic acid. In a real natural water system, reductants other than fulvic acid may be of importance. The results obtained in the laboratory, however, provide evidence that the Fe(OH)3(s)/Fe(II) redox couple is able to act as an electron-transfer mediator for the oxidation of natural organic substances, such as fulvic acid by molecular oxygen either in the absence of microorganisms or as a supplement to microbial activity.
Similar content being viewed by others
References
Berner, R. A., 1981. A new geochemical classification of sedimentary environments. J. Sediment Petrol. 51:359–365.
Buffle, J., R. R. De Vitre, D. Perret, G. G. Leppart, 1988. Physico-chemical characteristics of a colloidal iron phosphate species formed at the oxic-anoxic interface of a eutrophic lake. Geochim. Cosmochim. Acta 53:399–408.
Davison, W., 1985. Conceptual models for transport at a redox boundary. In: W. Stumm (ed.), Chemical Processes in Lakes, Wiley-Interscience, New York, pp. 31–53.
Davison, W., S. I. Heaney, J. F. Talling, E. Rigg, 1980. Seasonal transformation and movements or iron in a productive English lake with deep-water anoxia. Schweiz. Z. Hydrol. 42:196–224.
Deng, Y., 1992. Formation and dissolution of aquatic iron(III) (hydr)oxides — Implications for redox cycling of iron in natural waters. Ph. D. Thesis, ETH Zürich, Nr. 9724.
Deng, Y., W. Stumm, 1992. Reactivity of Aquatic Iron(III) (hydr)oxides — Implications for redox cycling of iron in natural waters. Applied Geochemistry (submitted).
Dos Antos, M., W. Stumm, 1992. Reductive dissolution of iron(III) (hydr)oxides by hydrogen sulfide. Langmuir. 8:1671–1675.
Ghiorse, W. C., 1988. Microbial reduction of manganese and iron. In: A. J. B. Zehnder (ed.), Biology of Anaerobic Microorganisms, Wiley-Interscience, New York, pp. 305–331.
von Gunten, U., W. Schneider, 1991. Primary products of the oxygenation of Fe (II) at an oxic-anoxic boundary: nucleation, aggregation and aging. J. Colloid Interface Sci. 145:127–139.
Lakind, J. S., A. Stone, 1989. Reductive dissolution of goethite by phenolic reductants. Geochim. Cosmochim. Acta 53:961–971.
Lovley, D. R., E. J. P. Phillips, D. J. Lonergan, 1991. Enzymatic versus nonenzymatic mechanisms for Fe(III) reduction in aquatic sediments. Environ. Sci. Technol. 25:1062–1067.
Ottow, J. G. G., A. von Klopotek, 1969. Enzymatic reduction of iron oxide by Fungi. Applied Microbiol. 18:41–43.
Schneider, W., B. Schwyn, 1987. The hydrolysis of iron in synthetic, biologcal, and aquatic media. In: W. Stumm (ed.), Aquatic Surface Chemistry, Wiley-Interscience, New York, pp 167–194.
Siffert, C., B. Sulzberger, 1991. Light-induced dissolution of hematite in the presence of oxalate: A case study. Langmuir 7:1627–1634.
Sørensen, J. and B. B. Jørgensen, 1987. Early diagenesis in sediments from Danish coastal waters: Microbial activity and Mn-Fe-S geochemistry. Geochim. Cosmochim. Acta 51:1583–1590.
Stumm, W., G. F. Lee, 1961. Oxygenation of ferrous iron. Industrial and Engineering Chem. 53:143–146.
Stumm, W., J. J. Morgan, 1981. Aquatic Chemistry, 2nd Ed., Wiley-Interscience, New York.
Sung, W. G., J. J. Morgan, 1980. Kinetics and products of ferrous iron oxygenation in aqueous systems. Environ. Sci. Technol. 14:561–568.
Suter, D., S. Banwart, W. Stumm, 1991. Dissolution of hydrous iron(III) oxides by reductive mechanisms. Langmuir 7:809–813.
Szilagyi, M., 1971. Reduction of Fe3+ ion by humic acid preparations. Soil Sci. 111:233–235.
Tamura, H., K. Goto, T. Yotsuyanagi, M. Nagayama, 1974. Spectrophotometric determination of iron(III) with 1,10-phenanthroline in the presence of large amounts of iron(III). Talanta 21:318–321.
Thurman, E. M., R. L. Malcolm, 1983. Structural study of humic substances: new approaches and methods. In: R. F. Christman and E. Gjessing (eds.), Aquatic and Terrestrial Humic Materials, Ann Arbor, Michigan, pp. 1–23.
Waite, T. D., F. M. M. Morel, 1984. Photoreductive dissolution of colloidal iron oxides in natural waters. Environ. Sci. Technol. 18:860–868.
Wehrli, B., E. Wieland, G. Furrer, 1990. Chemical mechanisms in the dissolution kinetics of minerals; the aspect of active sites. Aquatic Sci. 52:3–31.
Wersin, P., 1990. The Fe(II)-CO2-H2O system in anoxic natural waters: Equilibria and surface chemistry. Ph. D. thesis, ETH Zürich, Nr. 9230.
Wersin, P., P. Höhener, R. Giovanoli, W. Stumm, 1991. Early diagenetic influences on iron transformations in a freshwater lake sediment. Chem. Geol. 90:233–252.
Wells, M., E. D. Goldberg, 1991. Occurrence of small colloids in sea water. Nature 353:342–344.
Wieland, E., B. Wehrli, W. Stumm, 1988. The coordination chemistry of weathering: III. Generalization on the dissolution rates of minerals. Geochim. Cosmochim. Acta 52:1969–1981.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Deng, Y., Stumm, W. Kinetics of redox cycling of iron coupled with fulvic acid. Aquatic Science 55, 103–111 (1993). https://doi.org/10.1007/BF00877439
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00877439