Summary
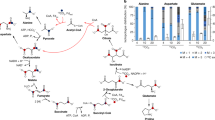
Photosynthesis (assimilation) is currently understood as a photoreduction of CO2 by water into carbohydrates; reoxidation by O2 (dissimilation) closes the biological carbon cycle. If, originally, there was a surplus of H2, thermodynamical equilibrium would require that C, Fe, Mn, S and N exist as CH4, Fe++, Mn++, S−− and NH3. The redox equilibrium is believed to have been shifted gradually as a result of the escape of H2 formed photochemically in the atmosphere. However, in a system containing H2, CO2 is an energy-rich compound affording 31.2 kcal on reduction to CH4; it should be so rare that even CaCO3 would dissolve. How, then, could photoreduction evolve, lacking CO2 and a thermodynamical advantage? Also H2 escape, governed by diffusion, does not account for so much H2 during geological time (Miller andUrey [19]). The hypothesis of areductive primeval carbon cycle between biomass (C(H2O)) and methane involvinginverse assimilation and dissimilation (Decker [6]), as presented in the following scheme, would evercome these difficulties. The present oxidative carbon cycle requires 114 kcal supplied by two consecutive photoreactions: regular:
A reductive carbon cycle involving inverse assimilation and dissimilation would require only 32 kcal, i.e. only one photoreaction: inverse:
It could actively support an elevated H2 level necessary to explain the observed escape rate, until all CH4 was consumed and burried as elemental C or carbonate, the latter resulting from fermentations in ‘anaerobic’ environments. All enzymatic steps of such a cycle are present in contemporary organisms except a photochemical CH4 assimilation in a CO2-free atmosphere, which, however, has apparently never seriously been looked for. Further facts pro and contra should be available in the geological record, e.g. in Precambrian ‘anaerobic’ Fe3+ and CaCO3 deposits.
Similar content being viewed by others
References
P. H. Abelson,Chemical events on the primitive Earth, Proc. Nat. Acad. Sci. (New York)55 (1966), 1365.
J. L. Bada andS. L. Miller,Ammonium ion concentration in the primitive ocean, Science159 (1968), 423.
W. H. Baur,Occurrence of nitride nitrogen in silicate minerals, Nature225 (1972), 461.
L. V. Berkner andL. C. Marshall,The rise of oxygen in the Earth's atmosphere with notes on the Martian atmosphere, Adv. Geophys.12 (1967), 309.
J. Brooks andG. Shaw,Origin and Development of Living Systems (Academic Press, New York 1973).
P. Decker,Inverse assimilation and dissimilation, possible metabolic patterns in a methane and hydrogen containing atmosphere, inProgress in Photosynthesis Research, edited byH. Metzner, Vol. I, p. 458 (Tübingen 1969).
P. Decker,Evolution in open systems: Bistability and the origin of molecular asymmetry, Nature New Biology241 (1973), 72.
P. Decker andA. Speidel,Open systems which can mutate between several steady states (‘Bioids’) and a possible prebiological role of the autocatalytic condensation of formaldehyde, Z. Naturforsch.27b (1972), 257.
P. Decker, A. Speidel andW. Nicolai,The origin of information in biological systems and in bioids, inProc. I Europ. Biophysics Congr., Baden, edited byE. Broda, Vol. IV, p. 515 (Verlag Wiener Med. Akad., Vienna 1971).
W. Ernst,Erdgas-Einwirkungen auf Pflanzen, Naturwissenschaften58 (1971), 620.
D. E. Fisher,Primordial rare gases in the deep Earth, Nature244 (1973), 344.
H. Gest,Comparative biochemistry of photosynthetic processes, Nature209 (1966), 879.
C. T. Gray andH. Gest,Biological formation of molecular hydrogen, Science148 (1965), 186.
J. B. S. Haldane,Origin of Life (Penguin Books, New Biology, London 1957).
N. H. Horowitz,On the evolution of biochemical syntheses, Proc. Nat. Acad. Sci. (New York)31 (1945), 153.
C. Junge,Die chemische Entwicklung der Erdatmosphäre, Umschau (1966), 767.
M. B. Kemp andJ. R. Quayle,Microbial growth on C 1 compounds, Biochem. J.102 (1967), 94.
E. Koch,Wasserpflanze überlebt in simulierter Jupiteratmosphäre, Umschau (1970), 216.
S. L. Miller andH. C. Urey,Organic compound synthesis on the primitive Earth, Science130 (1959), 245.
A. I. Oparin,Origin of Life (transl. by S. Margulis) (Macmillan, New York 1938).
I. Prigogine, G. Nicolis andA. Babloyantz,Thermodynamics of evolution, Physics Today (November 1972), 23.
S. I. Rasool andW. E. McGovern,Primitive atmosphere of the Earth, Nature212 (1966), 1225.
W. W. Rubey,Geologic history of sea water, inThe Origin and Evolution of Atmospheres and Oceans (Wiley, New York 1963), pp. 1–63.
H. Ruckert, E. Pfeil andG. Scharf,Über die Formaldehydkondensation III, der sterische Verlauf der Zuckerbildung, Chem. Berichte98 (1965), 2558.
M. Schidlowski,Probleme der atmosphärischen Evolution im Präkambrium, Geologische Rundschau60 (1971), 1351.
L. G. Sillen,Oxidation state of Earth's ocean and atmosphere I. A model calculation on earlier states. The myth of the ‘probiotic soup’, Arkiv för Kemi24 (1965), 431.
L. G. Sillen,Oxidation state of Earth's ocean and atmosphere II. The behavior of Fe, S and Mn in earlier states. Regulating mechanisms for O 2 and N 2, Arkiv för Kemi25 (1966), 159.
H. C. Urey,The atmospheres of the planets, Handbuch der Physik52 (1959), 363.
H. C. Urey, private communication (1969).
J. G. Zeikus andR. S. Wolfe,Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile, J. Bact.109 (1972), 707.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Decker, P. Inverse assimilation: Was the hydrogen escape from the earth's primary atmosphere enhanced by H2-evolution coupled with biophoto-oxidation of methane?. PAGEOPH 112, 865–875 (1974). https://doi.org/10.1007/BF00881492
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00881492