Conclusion
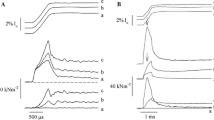
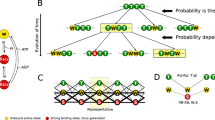
On the basis of measurements of the high energy phosphate usage associated with different mechanical states, as well as the degree of myosin light chain phosphorylation and mechanical properties, information has been gained concerning the existence and regulation of different crossbridge states in smooth muscle. Although incomplete, a general operational scheme is shown in figure 5. At very low intracellular calcium concentrations, actin and myosin are dissociated, as shown by a loss of resistance to stretch in resting muscles. At somewhat higher intracellular calcium concentrations in atonic, resting muscles, crossbridges can attach and be manifest mechanically as an increased resistance to stretch without ATP-driven crossbridge cycling and active force production. When the muscle is activated, intracellular calcium increases further, the light chains of myosin are phosphorylated through the calcium-calmodulin activation of myosin light chain kinase, actin-activated myosin ATPase activity increases and crossbridges cycle. Calcium also appears to modulate the ATPase activity and the rate of cycling of the phosphorylated crossbridge. The crossbridge cycling rate is highest during force development and slows with time as maximum isometric force is maintained reflecting a change in the rate at which phosphorylated crossbridges cycle. This may result from a decrease in the intracellular free calcium concentration with continued stimulation. During relaxation, the intracellular calcium concentration decreases, there is net dephosphorylation of the myosin light chains, the rate at which phosphorylated crossbridges cycle slows further with a gradual return to the attached, but non-cycling state or the detached state.
Similar content being viewed by others
References
Abbott, B.C., and Aubert, X.M., Changes of energy in a muscle during very slow stretches. Proc. R. Soc. Lond. B139 (1951) 104–126.
Abbott, B.C., Aubert, X.M., and Hill., A.V., The absorption of work by a muscle stretched during a single twitch or a short tetanus. Proc. R. Soc. Lond. B139 (1951) 86–117.
Aksoy, M.O., Mras, S., Kamm, K.E., and Murphy, R.A., Ca++ cAMP and changes in myosin phosphorylation during contraction of smooth muscle. Am. J. Physiol.245 (1983) C255–270.
Arner, A., Mechanical characteristics of chemically skinned guinea pig taenia coli. Pflügers Arch.395 (1982) 277–284.
Arner, A., Force-velocity relation in chemically skinned rat portal vein: Effects of Ca++ and Mg++. Pflügers Arch.397 (1983) 6–12.
Arner, A., and Hellstrand, P., Activation of contraction and ATPase activity in intact and chemically skinned smooth muscle of rat portal vein. Circulation Res.53 (1983) 695–702.
Ashton, F.T., Somlyo, A.V., and Somlyo, A.P., The contractile apparatus of vascular smooth muscle: intermediate high voltage electron microscopy. J. molec. Biol.98 (1975) 17–29.
Baguet, F., and Gillis, J. M., Energy cost of tonic contraction in a lamellibranch catch muscle. J. Physiol., Lond.198 (1968) 127–143.
Bozler, E., Mechanical Properties of Contractile Elements of Smooth Muscle, in: Physiology of Smooth Muscle, pp. 217ff. Eds E. Bulbring and M.F. Shuba. Raven Press, New York 1974.
Brutsaert, D.L., and Housmans, P.R. Load clamp analysis of maximal force potential of mammalian cardiac muscle. J. Physiol., Lond.271 (1977) 587–605.
Butler, T.M., Siegman, M.J., and Davies, R.E., Rigor and resistance to stretch in vertebrate smooth muscle. Am. J. Physiol.281 (1976) 1509–1514.
Butler, T.M., Siegman, M.J., Mooers, S.U., and Davies, R.E., Chemical energetics of single isometric tetani in mammalian smooth muscle. Am. J. Physiol.253 (1978) C1-C7.
Butler, T.M., and Siegman, M.J., Chemical energy usage and myosin light chain phosphorylation in mammalian smooth muscle. Fed. Proc.42 (1983) 57–61.
Butler, T.M., Siegman, M.J., and Mooers, S.U., Chemical energy usage during shortening and work production in mammalian smooth muscle. Am. J. Physiol.244 (1983) C234–242.
Butler, T.M., Siegman, M.J., and Mooers, S.U., Chemical energy usage during stimulation and stretch of mammalian smooth muscle. Pflügers Arch.401 (1984) 391–395.
Chacko, S., and Rosenfeld, A., Regulation of actin-activated ATP hydrolysis by arterial myosin. Proc. natn. Acad. Sci. USA79 (1982) 292–296.
Curtin, N.A., and Davies, R.E., Chemical and mechanical changes during stretching of activated frog skeletal muscle. Cold Spring Harb. Symp. Quant. Biol.37 (1973) 619–626.
Curtin, N.A., and Davies, R.E., Very high tension with very little ATP breakdown by active skeletal muscle. J. Mechanochem. Cell Motility3 (1975) 147–154.
Davies, R.E., Energy-rich phosphagens, in: Muscle metabolism during exercise, pp. 327–339. Eds B. Pernow and B. Saltin. Plenum Press, New York 1971.
Dillon, P.F., Aksoy, M.O., Driska, S.P., and Murphy, R.A., Myosin phosphorylation and the crossbridge cycle in arterial smooth muscle. Science.211 (1981) 495–497.
Edman, K.A.P., The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J. Physiol., Lond.291 (1979) 143–159.
Guth, K., and Mrwa, U., Maximum force is generated in chemially skinned taenia coli at lower Ca++ concentrations than maximum ATPase activity. Pflügers Arch.394 (1982) R44 (abstract).
Hellstrand, P., and Johansson, B., The force-velocity relation in phasic contractions of venous smooth muscle. Acta physiol. scand93 (1975) 157–166.
Hill, A.V., and Howarth, J.V., The reversal of chemical reactions in contracting muscle during an applied stretch. Proc. R. Soc. Lond. B151 (1959), 169–193.
Infante, A.A., Klaupiks, D., and Davies, R.E., Adenosine triphosphate: Changes in muscles doing negative work. Science144 (1964) 1577–1578.
Lowy, J., and Hanson, J., Ultrastructure of invertebrate smooth muscle. Physiol. Rev.42 (1962) 34–47.
Maréchal, G., Le métabolisme de la phosphorylcréatine et de l'adenosine triphosphate durant la contraction musculaire. Arscia, Brussels 1964.
Nag, S., and Seidel, N.C., Dependence on Ca++ and tropomyosin of the actin-activated ATPase activity of phosphorylated gizzard myosin in the presence of low concentrations of Mg++. J. biol. Chem.258 (1983) 6444–6449.
Paul, R.J., Doerman, G., Zeugner, C., and Ruegg, J.C., The dependence of unloaded shortening velocity on Ca++, calmodulin and duration of contraction in ‘chemically skinned’ smooth muscle. Circulation Res.53 (1983) 342–351.
Siegman, M.J., Butler, T.M., Mooers, S.U., and Davies, R.E., Calcium-dependent resistance to stretch and stress relaxation in resting smooth muscles. Am. J. Physiol.231 (1976) 1501–1508.
Siegman, M.J., Butler, T.M., Mooers, S.U., and Davies, R.E., Mechanical and energetic correlates of isometric relaxation in mammalian smooth muscle, in: Excitation-Contraction Coupling in Smooth Muscle, pp. 449ff. Eds R. Casteels, T. Godfraind and J.C. Ruegg. Elsevier/North Holland Biomedical Press, Amsterdam 1977.
Siegman, M.J., Butler, T.M., Mooers, S.U., and Davies, R.E., Chemical energetics of force development, force maintenance and relaxation in mammalian smooth muscle. J. gen. Physiol.76 (1980) 609–629.
Siegman, M.J., Butler, T.M., Mooers, S.U., and Michalek, A., Ca++ can affect Vmax without changes in myosin light chain phosphorylation in smooth muscle. Pflügers Arch.401 (1984) 385–390.
Uvelius, B., Shortening velocity, active force and homogeneity of contraction during electrically evoked twitches in smooth muscle. Acta physiol. scand106 (1979) 481–486.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Siegman, M.J., Butler, T.M. & Mooers, S.U. Energetics and regulation of crossbridge states in mammalian smooth muscle. Experientia 41, 1020–1025 (1985). https://doi.org/10.1007/BF01952125
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01952125