Summary
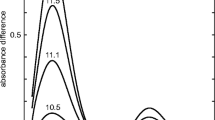
The rate of development of Ruhemann's purple in the ninhydrin reaction of two deuterated primary amines, αα-d2-p-tyramine and αα-d2-β-phenylethylamine, is significantly reduced It appears to be a primary isotope effect and indicates that the cleavage of the carbon-hydrogen bond at the α-position is involved in the rate-determining step of the color reaction.
Similar content being viewed by others
References
S.J. Ruhemann, J. chem. Soc.99, 1486 (1911).
D.J. McCaldin, Chem. Rev.60, 39 (1960).
P.H. Yu, S. Barclay, B.A. Davis and A.A. Boulton, Biochem. Pharmac.30, 3089 (1981).
S. Moore, J. biol. Chem.243, 6281 (1968)
S. Moore and W.H. Stein, J. biol. Chem.176, 367 (1948).
Author information
Authors and Affiliations
Additional information
Acknowledgements. We thank Dr A.A. Boulton for his advice and encouragement and the Canadian Medical Research Councel and Saskatchewan Health for their continuing financial support.
Rights and permissions
About this article
Cite this article
Yu, P.H., Davis, B.A. Deuterium isotope effects in the ninhydrin reaction of primary amines. Experientia 38, 299–300 (1982). https://doi.org/10.1007/BF01949352
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01949352