Climate Change Impacts on Seagrass Meadows and Macroalgal Forests: An Integrative Perspective on Acclimation and Adaptation Potential
- 1Marine and Environmental Sciences Centre, Faculty of Sciences of the University of Lisbon, Lisbon, Portugal
- 2Interdisciplinary Centre of Marine and Environmental Research of the University of Porto, Matosinhos, Portugal
- 3Guia Marine Laboratory, Marine and Environmental Sciences Centre, Faculty of Sciences, University of Lisbon, Cascais, Portugal
- 4Plant Functional Genomics Group, Departamento de Biologia Vegetal, Biosystems and Integrative Sciences Institute, Faculdade de Ciências, Universidade de Lisboa, Lisbon, Portugal
- 5Norwegian Institute of Bioeconomy Research, Bodø, Norway
- 6Evolutionary Ecology of Marine Fishes, GEOMAR Helmholtz Centre for Ocean Research Kiel, Kiel, Germany
- 7CCMAR - Centre of Marine Sciences, CIMAR – Associated Laboratory, University of Algarve, Faro, Portugal
- 8Department of Zoology, Faculty of Sciences and Technology, Marine and Environmental Sciences Centre, University of Coimbra, Coimbra, Portugal
- 9Red Sea Research Center, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia
- 10Molecular Ecology Group, Faculty of Biosciences and Aquaculture, Nord University, Bodø, Norway
Marine macrophytes are the foundation of algal forests and seagrass meadows–some of the most productive and diverse coastal marine ecosystems on the planet. These ecosystems provide nursery grounds and food for fish and invertebrates, coastline protection from erosion, carbon sequestration, and nutrient fixation. For marine macrophytes, temperature is generally the most important range limiting factor, and ocean warming is considered the most severe threat among global climate change factors. Ocean warming induced losses of dominant macrophytes along their equatorial range edges, as well as range extensions into polar regions, are predicted and already documented. While adaptive evolution based on genetic change is considered too slow to keep pace with the increasing rate of anthropogenic environmental changes, rapid adaptation may come about through a set of non-genetic mechanisms involving the functional composition of the associated microbiome, as well as epigenetic modification of the genome and its regulatory effect on gene expression and the activity of transposable elements. While research in terrestrial plants demonstrates that the integration of non-genetic mechanisms provide a more holistic picture of a species' evolutionary potential, research in marine systems is lagging behind. Here, we aim to review the potential of marine macrophytes to acclimatize and adapt to major climate change effects via intraspecific variation at the genetic, epigenetic, and microbiome levels. All three levels create phenotypic variation that may either enhance fitness within individuals (plasticity) or be subject to selection and ultimately, adaptation. We review three of the most important phenotypic variations in a climate change context, including physiological variation, variation in propagation success, and in herbivore resistance. Integrating different levels of plasticity, and adaptability into ecological models will allow to obtain a more holistic understanding of trait variation and a realistic assessment of the future performance and distribution of marine macrophytes. Such multi-disciplinary approach that integrates various levels of intraspecific variation, and their effect on phenotypic and physiological variation, is of crucial importance for the effective management and conservation of seagrasses and macroalgae under climate change.
Climate Change Impact on Marine Macrophytes
Burning of fossil fuels since the eighteenth century Industrial Revolution increased the atmospheric CO2 concentration from a pre-industrial level of 280 ppm to >400 ppm (reached in 2013), a level that has not been reached over the past few million years (Monastersky, 2013). Increasing levels of CO2 enhance the greenhouse effect, trapping more solar radiation near the earth surface, which causes an increase in global temperatures (Keller, 2009). About 80% of the excessive heat is absorbed by the ocean. Consequently, average global ocean temperatures have increased by 0.9°C in the upper 700 m during the twentieth century (Domingues et al., 2008), and currently (2001–2005 average) rank among the highest levels recorded during the past 1.4 million years (Hansen et al., 2006). Concomitantly, ocean uptake of atmospheric CO2 leads to ocean acidification (Doney et al., 2009). Further consequences of rising temperatures are ranging from changes in atmospheric and ocean circulation, over changes in season succession, as well as in storm and precipitation patterns, to drought periods and altered thermal environments (Reay et al., 2007; Poloczanska et al., 2013). A cascade of extreme thermal events became particularly evident in the last years, with severe increases in both frequency and intensity (Reay et al., 2007; Field et al., 2012), and affected phenological cycles in both adult forms and early-life stages of many marine organisms (Poloczanska et al., 2013). Summer warm extremes have increased by about 10% since the 1960's to 1970's in China and Europe (Yan et al., 2002; Klein Tank et al., 2003; Alexander et al., 2006) and the European heat waves in summer 2003 and 2010 (Beniston and Stephenson, 2004; Schär and Jendritzky, 2004; Barriopedro et al., 2011) caused major community shifts and local species extinctions (e.g., Garrabou et al., 2009; Sorte et al., 2010). Increasing ocean temperature and changing chemistry affects physiological performance, behavior, and population dynamics of all marine organisms, from primary producers to upper-trophic-levels, including fishes, seabirds, and marine mammals (Doney et al., 2012).
With the exception of hydrothermal vents in the deep sea, photosynthetic primary producers are at the base of all food webs. Here, we focus on macrophytes as key primary producers in marine benthic habitats, commonly known as seagrasses (marine angiosperms) and brown macroalgae. Seagrasses are Archaeplastida, the primordial photosynthetic eukaryote group which includes also green and red algae. In contrast, brown macroalgae are Stramenopiles (SAR), a lineage that gained chloroplasts in some groups by secondary endosymbiosis from other eukaryotes. Brown macroalgae (hereafter referred to as macroalgae) include fucoids that grow mainly in the intertidal, and kelps, a term used to designate large subtidal brown algae, most with a heteromorphic life cycle in the orders Laminariales, Tilopteridales, and Desmarestiales. An exception is bull kelp that is classified as a fucoid. Taxonomic understanding of both groups remains incomplete and in need of further refinement (reviews Hartog and den Kuo, 2006; Bartsch et al., 2008; Bolton, 2010), despite recent advances (Lane et al., 2006; Aires et al., 2011; Coyer et al., 2013; Rothman et al., 2015, 2017; Jackson et al., 2017). The continued application of genome-wide markers and multigene phylogenies will likely reveal previously overlooked taxonomic and biogeographic lineages (e.g., Tellier et al., 2009, 2011).
Both seagrasses and brown macroalgae are not only key primary producers, but also foundation species that influence ecosystem structure and function by creating locally stable conditions and habitat for other species, while supporting some of the most productive and diverse coastal marine ecosystems on the planet (Costanza et al., 1997; Spalding et al., 2007; Chung et al., 2011; Smale et al., 2013; Thomson et al., 2015; Teagle et al., 2017). Marine macrophytes further provide ecosystem services, such as food for invertebrates and fish, a blue carbon sink, nutrient fixation, and protection of the coastline from erosion (Procaccini et al., 2007; Harley et al., 2012). While macroalgae predominate on rocky shores in temperate to polar regions (Steneck et al., 2002; Bolton, 2010), seagrasses predominate on sandy shores from temperate to tropical regions (Short et al., 2007).
Seagrass beds and kelp forests are increasingly threatened by a variety of stressors (Orth et al., 2006; Waycott et al., 2009; Krumhansl et al., 2016). The combined effect of multiple climate-change related stressors on the extinction risk and productivity of macrophytes can be additive, synergistic, or antagonistic (Wahl et al., 2011, 2015), and may not be predicted from the individual effect of each variable operating in isolation (Darling and Côté, 2008). Nevertheless, for many marine macrophytes, temperature is the most important range limiting factor, and ocean warming is considered the most severe threat among global climate change factors (Diaz-Almela et al., 2007; Moore et al., 2012; Jueterbock et al., 2013; Araújo et al., 2016; Assis et al., 2017a; Repolho et al., 2017). In contrast, the predicted rise in ocean CO2 concentration is likely to have a positive effect on growth and photosynthesis because most macrophytes are carbon-limited at current ocean dissolved inorganic carbon (DIC) (Koch et al., 2013). However, the effect is unlikely to be big since the predicted long-term rise in CO2 falls several orders of magnitude below current CO2 and pH fluctuations within seagrass beds and kelp forests (Saderne et al., 2013; Wahl et al., 2017). Thus, in this review we mainly focus on ocean warming as the most important climate-change effect.
For macroalgae, the magnitude and direction of abundance changes vary strongly between geographic regions (Krumhansl et al., 2016), but macrophyte losses are concentrated in warm-temperate to tropical regions (Nicastro et al., 2013; Fraser et al., 2014). Physiological, genetic, and modeling data predict, and already document, that rising temperatures cause massive die-offs of genetically unique populations along warm-temperate distribution limits and open up new thermally suitable habitat in polar regions (Wernberg et al., 2011; Jueterbock et al., 2013, 2016; Brodie et al., 2014; Krause-Jensen and Duarte, 2014; Valle et al., 2014; Olesen et al., 2015; Assis et al., 2016a, 2017a; Hyndes et al., 2016). How fast and far warm-temperate range edges will retract toward higher latitudes largely depends on the macrophytes' ability to rapidly acclimatize or adapt to warm temperature extremes. In contrast, how fast and far poleward range-edges will extend into polar regions does not only depend on suitable temperatures for reproduction, but also on the macrophytes' ability to adapt to the extreme polar light conditions with month-long winters of constant darkness, and month-long summers of constant light (Krause-Jensen and Duarte, 2014; Berge et al., 2015).
The aim of the present paper is to review the potential of marine macrophytes to acclimatize and adapt to major climate change effects via three pillars of intraspecific variation (Figure 1). A holistic picture of ecologically and evolutionary relevant variation integrates genetic variation (A1) with non-genetic mechanisms, involving the functional composition of the epigenome (A2) and the microbiome (A3). All three levels create plasticity and adaptability via phenotypic variation. Most important in a climate change context is physiological variation (B1), variation in propagation success (B2), and in biotic interactions (B3). We do not aim to review ecological effects on macrophyte associated ecosystems, but on the macrophytes themselves. Our ultimate goal is to provide insight into recent and novel approaches that might be integrated in multidisciplinary studies and integrative niche modeling approaches (C) toward a better understanding of the future of these foundation species in a changing world (Figure 1).
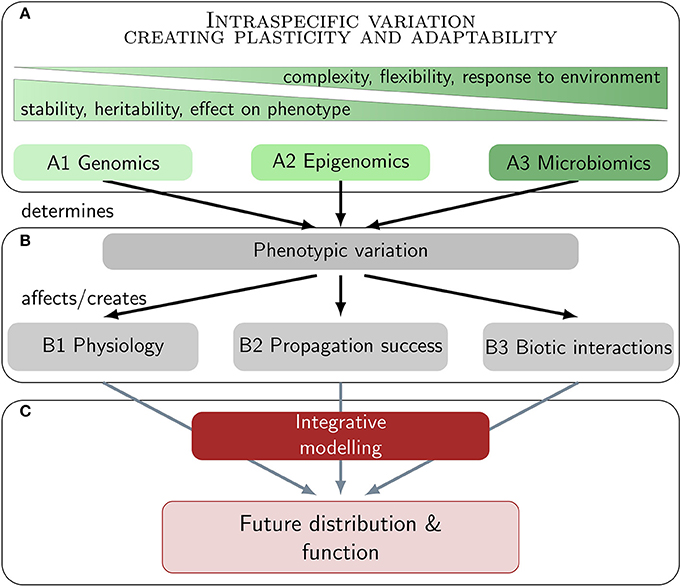
Figure 1. Realistic predictions of future distributions and ecosystem functions of marine macrophytes under climate change rely on multi-disciplinary research. Climate change research should ideally integrate various levels of intraspecific variation and their effect on phenotypic and physiological variation. The ultimate goal is to widen the concept of niche stability in conventional modeling approaches with this multi-layered plasticity concept.
A Levels of Intraspecific Variation Creating Plasticity and Adaptability
A1 Genetic Variation and Structure—Explained by Biogeographic History
Geographic patterns of neutral genetic structure in macroalgae and temperate seagrass species frequently reveal the imprints of ancient, often multiple refugia that arose during past glacial cycles and persist to the present day, revealing distinct genetic or phylo-groups sharing limited gene flow. In the Mediterranean, ancient vicariance events, hypothetically attributed to the Messinian Salinity Crisis, were reported in the seagrasses Posidonia oceanica (Arnaud-Haond et al., 2007b; Serra et al., 2010) and Ruppia spp. (Triest and Sierens, 2014), although niche modeling indicated that the present phylogeography of P. oceanica is also shaped by more recent climate refugia (Chefaoui and Serrão, 2017). Mediterranean-Atlantic vicariance with two glacial refugia in West Africa and the Eastern Mediterranean was suggested in Cymodocea nodosa based on allelic distributions (Alberto et al., 2008), and niche models (Chefaoui et al., 2017). Across latitudinal gradients, a recurring theme is genetically unique and rich low latitude rear edge populations and low-diversity poleward along post-glacial expansion fronts (e.g., for Zostera noltii, Coyer et al., 2004; Diekmann et al., 2005), while central latitudes can be genetically rich (Zostera marina; Diekmann and Serrão, 2012). Some works point out to strong impacts of climate change on seagrasses (Valle et al., 2014) and macroalgae (Wernberg et al., 2011; Jueterbock et al., 2013; Assis et al., 2016a,b, 2017a; Neiva et al., 2016), with poleward shifts at low diversity expansion fronts, while significant diversity near the rear edge may be lost.
A range of life-history and oceanographic features can serve to maintain existing population or metapopulation structure. Seagrass meadows often spread via vegetative clonal expansion, with individual clones reaching extreme age and extent in long-lived species (e.g., Posidonia oceanica, Arnaud-Haond et al., 2012 or Zostera marina Reusch et al., 1999). Clonal propagation creates challenges for genetic and biogeographical studies (Arnaud-Haond et al., 2007a), such as requiring high resolution genetic markers for identifying individuals. Notwithstanding the importance of sexual propagation for seagrasses (Kendrick et al., 2012), its principal role may be recolonization after disturbance (Marba and Duarte, 1995).
Kelps disperse via haploid meiospores, subsequently followed by syngamy between closely spaced (ca. 1 mm) benthic dioicous male and female microgametophytes. As fertilization occurs after the planktonic phase, low dispersal distance and inbreeding may be the norm (Reed et al., 2004b; Raimondi et al., 2011; Johansson et al., 2013), although km-scale dispersal is possible given a suitably large source population (Reed et al., 2004a).
On biogeographic scales, dispersal of viable vegetative fragments, larger-scale rafting, or sexual propagules is dependent on surface currents transporting viable material (Alberto et al., 2011; Johansson et al., 2015; Assis et al., 2017a). However, vicariance signatures suggest this type of dispersal is frequently unsuccessful in producing effective recruitment in seagrasses (Diekmann et al., 2005; Arnaud-Haond et al., 2007b; Alberto et al., 2008; Serra et al., 2010) but see Reusch et al. (2000) for Z. marina rafting success in the North Sea. A compelling hypothesis is that, even if oceanographic barriers are incomplete, dispersal into areas already colonized may be ineffective due to priority or density-barrier effects, in which incoming alleles are “swamped” by those already present at higher-frequency (De Meester et al., 2002; Neiva et al., 2012, 2016).
Populations differentiated along a latitudinal gradient experience climate change in different ways, due to their intrinsic genetic characteristics and population dynamics. A clearer picture of the biogeographic and metapopulation structure of macroalgae and seagrasses will be key to unravel variation in functional responses, adaptive potential, and likely resilience across species ranges. Much remains to be discovered, with the challenge to link genomic (Olsen et al., 2016) and functional trait variation (Jueterbock et al., 2016), and integrate this with projected threats arising from a rapidly changing climate. This is particularly urgent for rear-edge and marginal populations, many of which are under imminent threat.
Macrophytes can be locally adapted to their thermal regime (e.g., Zardi et al., 2013; Pereira et al., 2015; Saada et al., 2016; King et al., in press). This means that migrations are rather the dislocation of those adapted subpopulations, and must also be investigated/modeled as such (as in Assis et al., 2016b). Thus, below the surface of a species with an apparent broad tolerance, there may be more specialized sub-populations. However, the loss of genetic variability is likely to act against local adaptation of marginal populations (Pearson et al., 2009).
Genetic diversity is considered to be the key for future adaptation to environmental change, and the long-term survival of species (Bijlsma and Loeschcke, 2012). Genotypic diversity, as one subset of genetic diversity in clonally growing species, has been shown to increase productivity, and stress resilience in seagrasses and macroalgae (Hughes and Stachowicz, 2004; Reusch et al., 2005; Ehlers et al., 2008). Conservation management integrates the positive relation between genetic diversity and adaptive potential by focusing conservation efforts on populations with low genetic diversity, and by considering genetically diverse populations as source populations for restoration. However, evolutionary success of >1,000-years-old clonal seagrass beds with extremely low genetic diversity (Reusch et al., 1999) under substantial environmental change (Leipe et al., 2008), and the successful establishment of a putatively small North-European founder population of Laminaria hyperborea in Arctic Svalbard over the past few decades (Müller et al., 2009; Assis et al., 2016b) challenge the hypothesis of a straightforward relationship between genetic diversity and adaptive potential.
A2 Epigenetic Potential to Adapt to Climate Change
Epigenetic variation may contribute to rapid adaptation under climate change (Zhang et al., 2013; Schlichting and Wund, 2014; Prokopuk et al., 2015; Herman and Sultan, 2016; Rey et al., 2016), as adaptive evolution via DNA based polymorphisms is often considered too slow to keep pace with the increasing rate of anthropogenic environmental change (Quintero and Wiens, 2013). Epigenetic variations are molecular modifications that alter gene expression, but not the underlying DNA sequence, and occur in the form of histone modifications, non-coding RNAs, and DNA methylations (Bossdorf et al., 2008; Berger et al., 2009). Recent studies in the young research field of Ecological Epigenetics provide increasing evidence for the potential of epigenetic variation to increase plasticity, facilitate speciation and accelerate adaptation to new environments and stressful conditions (Schrey et al., 2013; Bonasio, 2015; Verhoeven et al., 2016; Kilvitis et al., 2017; Richards et al., 2017).
DNA-methylation variants (epialleles), epigenetic modifications that involve the addition of a methyl-group to cytosines (5-mC) in DNA sequence motifs, are currently the most popular epigenetic modification screened in evolutionary and ecological contexts (Schrey et al., 2013; Verhoeven et al., 2016; Richards et al., 2017). The possibility of methylation changes to respond directly to environmental change, and to trigger at least partly heritable changes in gene expression, resembles Lamarck's theory of the inheritance of acquired characteristics (Schmitz et al., 2013; Herman and Sultan, 2016) and ultimately challenges the classical theory of evolutionary adaptation.
The integration of epigenetic variation will certainly provide a more comprehensive understanding of the ecologically and evolutionary relevant variation of marine macrophytes, very much in the light of the recently suggested extended evolutionary synthesis (Pigliucci and Müller, 2010). This may enable for a more holistic prediction of the susceptibility of populations in terms of both genetic and epigenetic adaptive potential and, thus, for a more holistic conservation management under climate change. Due to their sessile nature, epigenetic variation is expected to be particularly relevant for rapid adaptation of marine macrophytes under climate change (Liu, 2013). Given the ecological key role of habitat-forming seagrass meadows, and macroalgal beds, epigenetic diversity in these systems is likely to secure the function of the entire associated coastal ecosystems. For example, DNA methylation enhanced the productivity, competitive advantage, and pathogen resistance of Arabidopsis thaliana plant populations (Latzel et al., 2013). Moreover, since the epigenome plays an essential role in plant development (Feng et al., 2010; Gutierrez-Marcos and Dickinson, 2012; Kawashima and Berger, 2014), its understanding is crucial to optimize seedling and gametophyte propagation for sustainable management, restoration, and cultivation of marine macrophytes.
Epigenetics research is growing particularly strong in terrestrial plants (Hirsch et al., 2013; Slotkin, 2016; Richards et al., 2017). A case in point within the context of climate change, is thermal tolerance that may be partly ascribed to CG methylation-variants, which is supported by temperature-associated variation in gene-body methylation in natural populations of A. thaliana (Dubin et al., 2015; Keller et al., 2016) and the valley oak Quercus lobata (Gugger et al., 2016). Moreover, experimental warming increased CHH methylation in A. thaliana transposable elements (Dubin et al., 2015) and contributed to increased methylation variation and adaptive plasticity in seedlings of the alpine herb, Wahlenbergia ceracea (Nicotra et al., 2015). However, these studies could not resolve to what extent the temperature associated methylation variants provided an autonomous way of adaptation that cannot be simply explained by underlying genetic variation (Foust et al., 2016; Herrera and Bazaga, 2016).
Epigenetic responses to climate change related stresses have to date been understudied in marine organisms (reviewed in Hofmann, 2017). One pioneering study on an Antarctic polychaete showed that a net increase in DNA methylation contributed to acclimation to warmer temperatures (−1.5° vs. +4°C), by regulating energy metabolism (Marsh and Pasqualone, 2014). In the European sea bass, a temperature increase of 2°C was shown to change global DNA methylation in larval but not in juvenile stages (Anastasiadi et al., 2017). In the scleractinian coral Pocillopora damicornis, DNA methylation levels increased globally in response to increased pCO2 levels (from ambient pH 7.9–7.65 to low pH 7.6–7.35) (Putnam et al., 2016). Accordingly, increasing DNA methylation likely contributed to phenotypic acclimation of the coral Stylophora pistillata under long-term exposure to reduced pH (Liew et al., 2017). These pioneering studies suggest that DNA methylation increases plasticity and adaptive potential in an ocean climate change context. While they focused on marine metazoans, the field is wide open in marine macrophytes.
Seagrasses likely show methylation in the same three sequence contexts as terrestrial plants, including CHG, CHH, and CG (Xie and Yu, 2015; Kilvitis et al., 2017; Richards et al., 2017). So-called CpG islands represent clusters of CG sites in gene promoter regions, and are commonly associated with gene expression regulation (Bossdorf et al., 2008; Illingworth and Bird, 2009; Zhang and Jeltsch, 2010). Transposable elements, generally silenced by DNA methylation, can be activated by stress-induced de-methylation processes and move to new genomic locations (Zhang et al., 2006; Slotkin and Martienssen, 2007; Biémont, 2010; Seymour et al., 2014). Such “jumping genes” can trigger changes in gene expression and genome structure, and may facilitate rapid species adaptation to new environmental conditions (González et al., 2010; Chénais et al., 2012; Casacuberta and González, 2013; Schrader et al., 2014; Stapley et al., 2015; Staton and Burke, 2015). Accordingly, a burst of transposable elements in the genome of the seagrass Zostera marina likely provided novel promoters and splicing sites, resulting in a gain of genes which may have facilitated its adaptation to the marine environment (Olsen et al., 2016). This suggests that DNA methylation changes and associated re-activation of transposable elements can additionally play an important role for rapid adaptation of seagrass under climate change.
Their partial clonal reproduction makes seagrass particularly well suited for epigenetic studies, as it allows to investigate epigenetic differentiation and change without the confounding factor of genetic variation. Vegetative reproduction entirely circumvents the meiotic resetting of epigenetic marks, thus enhancing the transgenerational epigenetic memory of clonal plants (Verhoeven and Preite, 2014; Latzel et al., 2016). Geographic variation in the degree of clonal reproduction allows to study the relevance of epigenetic variation under different reproduction modes. In a ~1,000-year old, predominantly clonal seagrass (Z. marina) meadow in the Baltic sea (Reusch et al., 1999), genetically identical shoots were recently shown to vary epigenetically in DNA methylation (Jueterbock et al., 2017). This variation may confer advantages that compensate evolutionary costs of clonal reproduction (Douhovnikoff and Dodd, 2014; Latzel et al., 2016), and may partly explain the evolutionary success of clonal seagrass meadows in the absence of genetic variation.
Another route to achieving genetic variation, despite clonal growth, may be somatic mutations, which have long been dismissed as route for adaptive mutational change. However, they do exist in long-lived seagrasses clones (Reusch and Boström, 2010) and may confer fitness advantages. Interestingly, in corals, genetic heterogeneity has also been observed within clones (Schweinsberg et al., 2015).
In contrast to seagrass DNA, it is still not clear which species of multicellular brown algae use DNA methylation as epigenetic mechanism. DNA methylation was found in the genomes of green and red algae, as well as in single-celled brown algae, and in diatoms (Maumus et al., 2011; Tirichine and Bowler, 2011; Veluchamy et al., 2013). MSAP analysis detected DNA methylation also in the kelp Saccharina japonica, with higher methylation-levels in sporophytes (ca. 25%) as compared with gametophytes (ca. 5%) (Qu et al., 2013). In contrast, undetectable 5-mC and C5-methyltransferase genes in the genome of Ectocarpus siliculosus (Cock et al., 2010) suggests a lack of DNA methylation as a derived feature in the brown algal order Ectocarpales. Sequence contexts and inheritance patterns of DNA-methylation variants are entirely unknown in brown macroalgae.
The first steps in marine macrophyte epigenetics can be taken with existing high-throughput techniques (Laird, 2010; Zhang and Jeltsch, 2010; Richards et al., 2017). Bisulfite sequencing is considered the 'gold-standard' to characterize DNA-methylation as the only method resolving nucleotide-level polymorphisms (Schrey et al., 2013; Adusumalli et al., 2014). While whole genome bisulfite sequencing is still expensive for large sample sizes, reduced representation techniques, such as RRBS (Gu et al., 2011), bsRADseq (Trucchi et al., 2016), and epiGBS (van Gurp et al., 2016) allow for cost-effective population epigenetic comparisons, partly without the need for a reference genome. Alternatively, DNA-methylation can be characterized with markers obtained via methylation-sensitive restriction enzymes, such as EpiRAD (Schield et al., 2016) and MethylRAD (Wang et al., 2015). While epigenetic population comparisons and stress responses can be studied for any species with DNA-methylation, an annotated reference genome is essential to infer the functional relevance of epigenetic differences and stress responses.
As numerous temperate macrophytes are predicted to shift poleward under projected climate change (see Biogeographic history section or niche modeling section), rapid acclimation and adaptation potential will become particularly relevant along their equatorial and polar distribution edges. Initial research priorities in marine macrophytes with respect to the relevance of epigenetic variation under climate change, are: (1) to characterize the relation between epigenetic and genetic variation and structure along latitudinal temperature gradients, and (2) to identify induced epigenetic changes in response to climate change related stress, their effect on TE activity and gene expression, as well as their heritability, and thus adaptive potential.
A3 Marine Macrophyte Holobionts and Their Hologenomes
In addition to the above covered mechanisms for adaptation and acclimation of marine macrophytes, we highlight in this section that acclimation can also be mediated by changes in the structure of associated microbial communities. These fast dividing and evolving members of microbial communities can change orders of magnitude faster than their host. Future climate change conditions can shift host microbiome structure (microbial composition and abundances) and function. To what extend the host influences these shifts and whether these shifts increase the hosts' fitness under changed conditions is uncertain, but if so acclimation could be microbiome-mediated (Webster and Reusch, 2017). Over the last decade, it has become increasingly clear that the fitness of macro-organisms is at least partially determined by their associated microbiota, the microbiome, consisting of archaea, bacteria, fungi, viruses, protists, etc., all together with the host forming a holobiont. In the marine realm, microbiomes have been studied especially in corals and sponges (Bourne et al., 2016; Keller et al., 2016; Hernandez-Agreda et al., 2017). Although macroalgae and seagrasses form habitats worldwide known as hotspots of biodiversity and production, we know little about the microbes in these ecosystems (Bengtsson et al., 2012), but see Clasen and Shurin (2015) for an ecosystem approach. The relative few microbial studies performed have focused almost exclusively on (epi-)bacterial communities associated to seagrasses and macroalgae, neglecting most other microbes (Bengtsson et al., 2012; Bockelmann et al., 2012, 2013; Michelou et al., 2013; Brakel et al., 2014, 2017; Cúcio et al., 2016; Singh and Reddy, 2016). On top of that, particularly functional interactions between marine macrophytes and their microbiomes are poorly known. Molecular ecology of seagrass and macroalgae microbiomes is a young research field. With the progress in high throughput sequencing, it has the potential to radically influence our understanding of seagrass and macroalgae ecology.
Marine macrophytes associate with bacterial communities that differ strongly from those of their surrounding seawater, sediment or substrate (Bengtsson and Øvreås, 2010; Bengtsson et al., 2010; Aires et al., 2016; Cúcio et al., 2016). However, bacterial communities associated to marine macrophytes are not fixed and can change temporally and spatially across seasons, lifespan, life stages and tissue types by biotic and abiotic factors (Staufenberger et al., 2008; Aires et al., 2016; Mancuso et al., 2016). While bacterial communities of some macroalgae appear species- or even lineage-specific (Aires et al., 2016; Vieira et al., 2016), this is yet unclear for kelps and seagrasses due to the low number of studies with inter-species comparisons. Recent results (Cúcio et al., 2016) suggest that sympatric seagrass species (in this case Z. marina, Z. noltei, and Cymodocea nodosa) might share largely the same rhizosphere community. Similar results were very recently obtained by (Crump et al., 2018) for a comparison between sympatric Z. marina and Z. japonica in Oregon, USA using metatranscriptomics and 16S amplicon sequencing. The co-occuring Halophila ovalis, Halodule uninervis, and Cymodocea serrulata each showed unique root microbiomes, as light was experimentally reduced their root exudation was altered which reduced the abundance of microorganisms that are potentially beneficial to the seagrasses, but not the predicted function (Martin et al., 2018).
Only few macroalgae have been the subject of microbiome studies. On L. hyperborea, one of Europe's most important marine forest former, epibacterial diversity increases with the age/successive colonization of the kelp surface (Bengtsson et al., 2012). Bacterial density and community composition follow the kelp seasonal growth cycle. As most of the biofilm seems to consist of bacteria utilizing carbon produced by the host (Bengtsson and Øvreås, 2010), microbiome dynamics are probably strongly linked to seasonal changes in the kelp metabolome and seawater temperature (Bengtsson et al., 2010). A recent study using shotgun metagenomics suggests a complementary and mutualistic relationship between the female gametophyte of the kelp Saccharina japonica and its microbiome, in which bacteria seem to benefit from kelp polysaccharides and the kelp profits from enhanced growth and nutrient uptake by bacterial bioactive compounds such as vitamins and hormones (Ji et al., 2017). Gene functions within this kelp epi-microbiome were mainly symbiosis-associated, indicating that selective pressures shape these microbiomes to sustain a mutual benefit for both kelp and bacteria (Ji et al., 2017).
Macroalgae associated bacteria can have a wide range of beneficial effects for their host. Already at a very early stage of development, macroalgae can depend on associated bacteria. Green algae of the genus Ulva, for example, depend on bacterial compounds for induction of cell divisions, differentiation, wall formation, and hence a normal morphogenesis (Provasoli and Pintner, 1980; Matsuo et al., 2003; Wichard, 2015; Grueneberg et al., 2016). We are not aware of similar findings reported for kelp or seagrasses. Kelp associated bacteria can have growth-promoting effects, like noted for L. japonica (Dimitrieva et al., 2006) and provide nutrients. In more than half of the algal kingdom bacteria provide vitamins to their hosts (Croft et al., 2005). Azotobacter bacteria associated with the green alga Codium fragile, for example, were shown to be involved in nitrogen fixation, and are thought to supply the alga with nitrogen compounds (Head, 1975). Diazotrophic heterotrophic bacteria in seagrasses rhizosphere are involved in nitrogen fixation (Welsh, 2000). Macrophyte associated bacteria are also involved in the production of biologically active and defensive compounds, protecting the host from pathogens, herbivores, fouling, and chemical intrusion (Burgess et al., 1999; Rao et al., 2007; Penesyan et al., 2009; Egan et al., 2014; Saha et al., 2014). Bacterial antimicrobial metabolites negatively affect fouling organisms and control microbial communities on macroalgae surfaces (Egan et al., 2000; Joint et al., 2007; Romero et al., 2011). In L. saccharina, half of the bacterial strains isolated by Wiese et al. (2009) demonstrated antimicrobial activity, inhibiting the growth of at least one Gram-negative and Gram-positive bacterium.
In the light of rising seawater temperatures and consequent stress conditions for seagrasses and macroalgae resulting in northward shifts of their species distribution, it is important to assess the role associated microbiomes can perform for their macrophytic host. Increased physical disturbances and stress, resulting from changing environmental conditions, are known to affect the macroalgae-associated microbial composition (directly or via its physiological responses) and cause its structural, functional or behavioral changes (Goecke et al., 2010; Egan et al., 2013; Hollants et al., 2013; Dittami et al., 2016). Microorganisms seem to be able to play a pivoting role in enabling macrophytes to expand their physiological capacities, broadening their environmental tolerance (Goecke et al., 2010; Egan et al., 2013; Hollants et al., 2013). In a species of the genus Ectocarpus for example, specific bacteria are linked to low salinity tolerance in Ectocarpus cultures facilitating acclimation to environmental change (Dittami et al., 2016). To what extend the marine macrophyte microbiome could be involved in thermal acclimation and adaptation is not known. In terrestrial plants, fungal symbionts increased plant biomass under various global change scenarios, including warming (Kivlin et al., 2013). In their review, the authors conclude that it is critical to include plant-fungal symbioses in the prediction of ecosystem response to global change (Kivlin et al., 2013). In some cases, however thermal tolerance involves more partners. Marquez et al. (2007) showed elegantly that a virus of a fungal endophyte of a tropical grass confers heat tolerance to both organisms enabling them to grow at high soil temperatures. Experimental warming of Cymodocea nodosa and Labyrinthula spp. that cause seagrass wasting disease, showed that the seagrass was not more susceptible to infection at higher temperature. On the contrary, lesion size decreased with warming (Olsen et al., 2014). Based on these cases it seems likely that also the macrophyte microbiome is linked to environmental tolerance, including warming. The only study that combined elevated temperatures and ocean acidification showed that elevated temperatures alone drives dysbiosis in Macrocystis pyrifera under which kelp growth was also negatively affected (Minich et al., 2017). However, acidification counteracted the elevated temperature effects resulting in positive kelp growth and a commensal microbial community that increased mucus production (Minich et al., 2017). Although it is not clear whether the microbiome changes in reaction to a change in the health of the host or vice versa (probably a tight and complex interaction of the two), it is increasingly clear that the interaction of global change factors on marine macrophytes and their microbiomes should be more investigated.
The association with microorganisms may allow marine macrophytes to acclimate/adapt to warming conditions by changing the composition of microbiota, a process that can be much faster than by genomic evolution. Therefore, the diverse microbiota can possibly assist the macrophyte holobionts functioning and survival under elevated temperatures. As the combination of host and microbiota genomes, i.e., the hologenome, might in some cases act as a unit of natural selection, the association with microbes might play a crucial role in the acclimation of marine macrophytes to warming. At this moment, few studies address this issue in seagrasses and macroalgae. However some evidences are already documented in filamentous algae (Dittami et al., 2016). Holobiont evolution requires strict partner fidelity and will only work under vertical transmission fidelity and can be evolutionary unstable due to microbial cheaters, or shifting cost:benefit ratios (Douglas and Werren, 2016). However, the persistence of co-introduced symbiont bacteria many decades after macroalgae invaded in the Mediterranean from Australia, and their correlation with the host ecology (Aires et al., 2013; Arnaud-Haond et al., 2017), suggests a tight inter-dependence in at least some invasive macroalgae lineages. Notwithstanding, positive functional roles of the host-associated microbiome are also possible if the microbiome and its host do not evolve as a strict unit. Thus, we propose that concepts of climate change effects on macroalgae and seagrasses require the inclusion of microbiome-mediated acclimation. In the coral world, the study of fitness effects of associated microbes is far more developed than in seagrasses and macroalgae. As some corals are functionally plants (i.e., in corals with photosynthetic symbionts photosynthesis mostly exceeds heterotrophic nutrition) it may be useful to actively search parallels among both functional groups of habitat forming species. For example, both somatic mutations and epigenetic changes owing to environmental conditions have recently been described, making corals a poster child for the adaptation of the holobiont (Webster and Reusch, 2017).
B Forms and Effects of Phenotypic Variation
B1 Physiological Constrains Promoted by Climate Change
The direct link between epigenetic mechanisms and gene expression (Bossdorf et al., 2008; Berger et al., 2009), and the proven effect of the root microbiome on the leaf metabolome in a terrestrial plant (Badri et al., 2013), demonstrate the direct relation between epigenetic or microbome variation and physiology. Despite the lack of research in marine macrophytes, it can be expected that rapid shifts in epigenetic marks or in the microbiome composition may contribute to acclimatization to climate-change related stressors.
Photosynthesis is one of the most essential physiological processes that will be affected by increasing ocean temperatures. From a physical point of view, increasing water temperatures reduce oxygen solubility and CO2 availability (Beardall et al., 1998). Photosynthetic efficiencies are severely impaired by low light and/or high temperature conditions (York et al., 2013). Kinetically, photosynthesis increases with increasing temperatures until an optimum temperature point or range of a few degrees, beyond which it declines rapidly (Davison, 1991). These physiological imbalances increase respiratory activity, and shift marine forests and seagrass beds from carbon sinks to carbon sources (York et al., 2013). Most photosynthetic changes are due to damage at the chloroplast level (Repolho et al., 2017). Thermal-stress induced structural alterations at the photosystem II (PS II) reaction centers can lead to photoinhibition over several days (Campbell et al., 2006), so that the affected macrophytes depend on the respiration of their storage compounds. Moreover, thermal stresses have a very typical signature at the PS II level (Srivastava et al., 1997; Strasser et al., 2000). Thermal stress impairs typically the donor side of the PS II reaction centers, which corresponds to the location of oxygen evolving complexes (OEC) (Strasser et al., 2000; Duarte et al., 2015a,b, 2016). This imposes serious physiological constrains to the chloroplastidial electron transport and energy production. Therefore, only species with a high degree of physiological plasticity can cope with abrupt changes, such as heat waves (Duarte et al., 2015a,b, 2016). From an ecological point of view, rising temperatures affect the role of kelp forests and seagrass beds as primary oxygen producers and, thus, all the heterotrophic food chain. Zhang et al. (2017) suggest four possible mechanisms in macrophytes to balance the redox state of electron transport and regulate the energy distribution between the two photosystems, thereby protecting the photosynthetic tissues from thermal stress: (1) an enhancement in the active PS II reaction centers efficiency; (2) an increase in the activity of the PS II electron acceptor side; (3) an enhancement in the cyclic electron flow transport around photosystem (PS I), allowing this photosystem to absorb the excessive electron flow; (4) alternation between PS II and PS I.
The accumulation of reducing power inside the chloroplast (Duarte et al., 2015a) increases non-photochemical quenching and decreases the photochemical quenching in order to dissipate excessive energy (Repolho et al., 2017). This can be performed by quantum dissipation or by enzymatic means, through the de-epoxidation of the xanthophylls (Figure 2, DES). Due to the characteristics of any enzymatic reaction, also this enzymatic dissipation mechanisms can be impaired outside the thermal optimum of each species (Campbell et al., 2006; Repolho et al., 2017). Additionally, ocean warming can lower chlorophyll contents and, thus, lead to kelp bleaching and seagrass browning (Staehr and Wernberg, 2009; Figure 2).
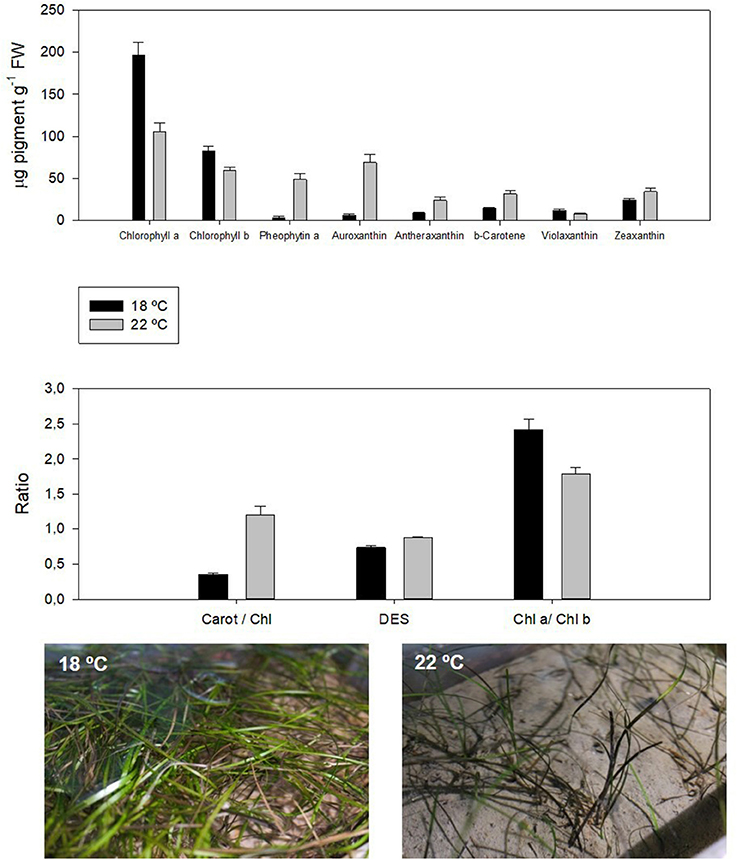
Figure 2. Pigment profile in the dwarf eelgrass (Zostera noltii) under control and ocean warming conditions. The decrease in chlorophylls and increase in carotenoid content as well as chlorophyll degradation products are symptoms of thermal stress, concomitant with the decrease in the chl a/chl b ratio, a well-known indicator of stress (adapted from Repolho et al., 2017).
Under stress, macrophytes can recycle the photosynthetic substrates using storage ATP and carbohydrates. Respiration is very sensitive to ocean warming. In Ecklonia radiata, respiration is more affected than photosynthesis, implying that increasing temperatures raise respiratory energy demands faster than the photosynthetic regeneration of new ATP and carbohydrates (Staehr and Wernberg, 2009). To prevent metabolic arrest under such conditions, the most direct means to maintain a positive carbon balance is to increase the light demands and the light harvesting capacity of the photosystems. In seagrasses, respiration mechanisms acquire a reinforced importance, due to the below-ground respiration of their root system. Leaf-based thermal optima may not represent thermal optima of the whole individual, since seagrasses have non-photosynthetic compartments (rhizome and roots), accounting for the majority of the total biomass (Collier et al., 2017). While respiratory activity under thermal stress can already surpass the plant photosynthetic capacity, this non-photosynthetic compartment imposes an additional respiratory burden (Fourqurean and Zieman, 1991).
The balance between photosynthesis and respiration will further determine whether macrophytes can extend poleward into regions that are predicted to become ice-free and thermally suitable. Survival of the light season in summer may depend on the ability for increased light-respiration under constant light conditions. In contrast, survival of the dark winter season depends on the ability to store enough photosynthetates during the summer months to compensate for constant respiration in month-long darkness (Berge et al., 2015), especially when energy metabolism increases with rising temperatures (McMinn and Martin, 2013).
Low oxygenation levels imposed by global warming alter the sediment microbial community and biogeochemistry in sediments, favoring anaerobic organic carbon oxidation (Holmer and Bondgaard, 2001). Although this metabolic shift allows the system to recycle organic carbon, it comes at a high cost by generating high amounts of sulfide that is toxic for seagrasses (Koch and Erskine, 2001). Seagrasses are able to tolerate sulfide intrusion by: (i) reoxidized sulfide by oxygen present in the aerenchyma (Pedersen et al., 2004) or in the rhizosphere (van der Heide et al., 2012) or (ii) organic sulfur conversion into thiols (Holmer and Hasler-Sheetal, 2014). If both mechanisms fail and sulfide intrudes into active tissues such as leaves and meristems, seagrasses suffer in performance (Garcias-Bonet et al., 2008; Pulido and Borum, 2010). Under increased anoxia, the already low levels of oxygen in the fine-grained soft sediments that seagrasses grow in, can increase sulfide production beyond optimal growth thresholds (Holmer and Bondgaard, 2001). Additionally, lower O2 availabilities can impair the biological sulfide reoxidation (Pedersen et al., 2004; van der Heide et al., 2012), leading to increased sulfide accumulation. Moreover, sulfide impairs the sucrose transport from leaves, where it is photosynthetically generated, to the other non-photosynthetic organs where it is stored or consumed for active growing (Holmer and Bondgaard, 2001). If the plant cannot translocate sucrose efficiently to feed the underground organs, it will consume the storage sugars (underground starch) to maintain root growth and nutrient acquisition metabolisms. This re-mobilization of underground starch shifts the plant metabolism from an energy producer to an active consumer and, thus, impairs growth and primary productivity (Holmer and Bondgaard, 2001; Koch and Erskine, 2001; Holmer and Hasler-Sheetal, 2014).
Other climate-change associated stressors, such as hurricanes and excessive precipitation, can be equally disturbing for macrophytes. One of the direct consequences of these extreme events is the alteration of sedimentary budgets in transitional and coastal waters. During flood events the export of sediment from the river catchments to estuarine basins is substantially increased (Duarte and Caçador, 2012). Although both seagrasses and kelps depend on the sediment budget for their anchorage, they experience increased turbidity as additional stress as it reduces light availability (De Boer, 2007; Saunders et al., 2017). Light availability is a key factor that contributes to about 75% of the variation in the distribution of seagrass meadows and kelp forests (De Boer, 2007). However, seagrasses and kelps also influence sedimentation by trapping sediments from the water column, and thus actively decrease turbidity (De Boer, 2007). The sensible equilibrium between unproblematic and problematic levels of water-borne sediments can be disturbed by other climatic variables that act as multiple, synergistic stressors, which trigger more extreme ecological responses, particularly in ecosystems where foundation species exist near upper thermal tolerance limits (Fraser et al., 2014). While acidification can to some extent alleviate thermal stress due to higher CO2 availability for photosynthesis (Repolho et al., 2017), exposure to multiple stresses may more often increase mortality and lead to carbon exportation.
Seagrass meadows and kelp forests can support high carbon uptake, depositing and preserving it as blue carbon over millennia in surrounding sediments (Duarte et al., 2013). The accelerated decline of macrophytes represents a loss of carbon sink capacity, and an increased risk for sedimentary carbon deposits to be lost through erosive and resuspension processes (Duarte et al., 2013). The loss of carbon stocks is not limited to carbon buried by erosion, but includes also the loss of carbon sink capacity and a potential functional shift from carbon sinks to carbon sources via the re-mobilization of carbon stocks accumulated over millennia (Duarte et al., 2013).
Among other energy storage molecules, fatty acids play an important role not only for the physiology of the plant itself, but also in terms of macrophyte-based trophic chains, which are either directly based on macroalgae and seagrasses as food-source or on detritus exportation (Duarte et al., 2017b; Repolho et al., 2017). While animals can produce metabolically unsaturated and monosaturated fatty acids, they are able to synthesize polyunsaturated fatty acids (PUFAs), like linoleic acid (C18:2, n-6) and α-linolenic acid (C18:3, n-3) (van Ginneken et al., 2011). These are precursors of long chain PUFAs (LC-PUFAs) such as arachidonic acid (C20:4, n-6), eicosapentaenoic acid (EPA, C20:5, n-3) and docosahexaenoic acid (DHA, C22:6, n-3). Ocean warming is expected to have strong directional effects on the quantity and quality of fatty acids in marine macrophytes (Hixson and Arts, 2016), which will lead to modifications of the structure of their cellular membranes (Winter and Dzwolak, 2005), a mechanism of acclimatization known as homeoviscous adaptation (Sinensky, 1974). This adaptation involves remodeling of membrane lipids via the modification of fatty acid chain length and saturation, allowing to maintain a desired level of fluidity in cell membranes (Sinensky, 1974; Guschina and Harwood, 2006; Matos et al., 2007; Feijão et al., in press), and counteracting the increased fluidity promoted by higher temperatures. The decrease in the number of double bonds in PUFA and the increase of saturated fatty acids (SFA), enhances the ability of fatty acids to maintain structural rigidity of cell membranes in a less ordered environment (Fuschino et al., 2011). Global warming is expected to reduce the global production of PUFAs by marine macrophytes (Hixson and Arts, 2016). These biochemical and physiological cascades are predicted to affect also terrestrial animals because of the flux of aquatic biomass, containing n-3 LC-PUFA, which normally passes from aquatic to terrestrial ecosystems (Gladyshev et al., 2013). Marine ecosystems provide essential LC-PUFA for many omnivorous terrestrial animals, including humans. This transport of essential LC-PUFA from sea to land occurs through the trophic chain, as most terrestrial consumers are directly or indirectly fed by ocean products (Gladyshev et al., 2013). PUFAs were shown to enhance growth rates and reproductive capacities of aquatic animals (Von elert, 2004), as well as to be of great importance to the neural/cognitive, cardiovascular, and visual health of terrestrial vertebrates (Calder, 2015). Any stress like warming, that affects the fatty acid composition of membrane lipids, has inevitable impacts on the photosynthetic and respiratory pathways that are already under stress due to perturbations at the energy transduction level (Matos et al., 2007; Gameiro et al., 2016; Duarte et al., 2017a).
B2 Propagation Success: Climate Change Impacts on Early Life Stages
In plants, epigenome and microbiome shifts play an essential role during the development of early life stages (Feng et al., 2010; Chaparro et al., 2014), the life stages that are generally most vulnerable to ocean warming in marine macrophytes (e.g., Brawley and Johnson, 1991; Schiel and Foster, 2006). Thus, propagation success of macroalgae and seagrass under climate change can be expected to at least partly depend on their epigenetic makeup or their microbiome composition.
Macroalgae, usually perceived as large canopy-forming beds or forests, all have microscopic phases that are generally highly susceptible to environmental stress (Roleda et al., 2007). The unicellular life stages of macroalgae, either spores or gametes, are released into the water column. These settle on the rocky coastline and give rise to the next macroscopic generation. Enormous numbers of these microscopic propagules are produced by macroalgae but only a small fraction survive to maturity. Physical stressors such as visible light, ultraviolet radiation (UVR), and temperature, account for much of the mortality among spores, embryos and juveniles (Schiel and Foster, 2006).
Macroalgae with complex life cycles have unicellular and microscopic cryptic stages that serve as seed banks for the next macroscopic generation. For example, the spores of some large macroalgae, such as kelps (i.e., Laminariales), germinate to produce a microscopic phase—a free-living generation with half the ploidy level of the macroscopic phase. Kelp sporophytes release spores that settle and germinate into free-living, haploid male and female microscopic filaments that grow on the seafloor. The gametophytes release sperm to fertilize eggs to form a zygote which develops into an embryonic sporophyte that matures into the morphologically complex macroscopic phase. Kelps are thus an example of a “heteromorphic alternation of generations” whereby two free-living phases are morphologically and ecologically distinct.
Stress physiological studies comparing the relative susceptibility of different life history stages showed that spores and gametes of brown, red and green macroalgae, are more susceptible to UVR compared to their corresponding juvenile and adult phases (Roleda et al., 2004, 2007, 2009). However, it seems that the microscopic kelp gametophytes are insensitive to anthropogenic CO2 induced ocean acidification (Roleda et al., 2012; Leal et al., 2017b), and relatively tolerant to ocean warming (Leal et al., 2017a). Examples of thermal tolerances are specific to species and even to populations, suggesting local adaptation.
Arctic kelps' spore photosynthesis and gametophyte growth rate have a high temperature affinity between 12 and 13°C (Roleda, 2009, 2016); this temperature is 7–8°C higher than the in situ summer water temperature (5–6°C) in Kongsfjorden (Svalbard) (Hanelt et al., 2001; Svendsen et al., 2002). Moreover, the germination rate was also enhanced when summer mean temperature was increased by 4–5°C (Zacher et al., 2016). Among cold temperate populations, photosynthesis of Laminaria digitata gametophytes from Roscoff (France) was also not compromised when average summer temperature (17°C) was increased by 3°C (Delebecq et al., 2016). Among kelps in the Pacific, spore germination of M. pyrifera from New Zealand was not affected while germling growth rate was enhanced by a 4°C increase in summer water temperature (Leal et al., 2017a). On the other hand, spore germination of the same species from California significantly decreased with a 5°C increase in temperature (Gaitán-Espitia et al., 2014).
Gametogenesis and fertilization are particularly sensitive to rising temperatures (Hooper, 1984; tom Dieck, 1989; Roleda, 2016). For example, the growth rate of Laminaria digitata gametophytes was highest at 10–18°C, while gametogenesis required lower temperatures (i.e., 10–15°C), and fertilization and recruitment of sporophytes was optimal at only 5°C (Martins et al., 2017).
Considering the worst-case scenario of a temperature increase by 4°C until 2100 (Reay et al., 2007), and predictions of more extreme warming in some areas in the northern hemisphere, photosynthesis and growth of different life history stages, e.g., the microscopic spores and gametophytes, and the canopy-forming sporophytes, are unlikely to be negatively compromised. However, ocean warming can negatively affect kelp's asexual (sporogenesis) and sexual (gametogenesis) reproduction (Viejo et al., 2011; Bartsch et al., 2013; Roleda, 2016; Martins et al., 2017). The impacts of climate change on the reproduction (both sporogenesis and gametogenesis) and embryogenesis among macroalgae with complex life cycles require further studies.
Cold temperatures currently prevent the dominant seagrass in the northern hemisphere, Zostera marina, to extend its range further poleward into Arctic regions, like Svalbard and northern Greenland. While this plant can flower at temperatures as cold as 0.5–3°C, the development of mature fruits requires 2 months at 14–15°C (Silberhorn et al., 1983). In contrast, extremely warm temperatures, like other stresses, commonly induce flowering in plants (Wada and Takeno, 2010). Accordingly, the Mediterranean seagrass Posidonia oceanica responded to an experimental heat wave (27°C for 6 weeks) with an up to 47% increase in flowering-rate (Ruiz et al., in press). While sexual reproduction certainly provides the potential to escape from too warm regions, and to increase genetic and phenotypic diversity in this highly clonal plant, this potential can only be realized if the produced early life stages can successfully establish a new generation.
Early life stages of seagrass are comprised by seeds and seedlings, which can be defined as the single shoot germinated from seed prior to initiation of clonal growth. Evidence for temperature effects on seagrass germination is equivocal, as some authors report enhanced germination rates with increasing temperature (e.g., Hootsmans et al., 1987; Jinhua et al., 2011; Kaldy et al., 2015), whereas others report no effects of temperature within the ranges explored (e.g., Phillips et al., 1983; Loques et al., 1990), even if some of these contrasting results refer to germination experiments conducted with the same species. These contrasting results reflect either local adaptation to particular spring regimes or the fact that the response of seagrass seed germination to temperature is likely to be best represented by a Gaussian distribution, with minimal and maximum thermal tolerances and an optimum temperature for germination. Abe et al. (2009) determined that optimal water temperature for seed germination of a Japanese Z. marina stand was in the range from 10 to 15°C, with optimal temperature for seedling growth ranging from 20 to 25°C; and seedling mortality observed at water temperatures exceeding 28°C. Based on these results, they predicted that Z. marina stands would be lost from areas exceeding maximum temperatures of 28°C (Abe et al., 2009), thereby leading to the expectation of losses with warming in the lower latitudinal limit of the species in Japan (Abe et al., 2009). Seed abortion following marine heat waves has been documented in Posidonia species, both in the Mediterranean (P. oceanica, Balestri and Cinelli, 2003) and Australia (P. australia, Shark Bay, Thomson et al., 2015).
Seedling performance is also negatively affected by extreme temperatures expected with further warming. Experimental simulated heat waves and warming at projected levels in the NW Mediterranean led to reduced growth, increased mortality, leaf necrosis, and respiration in Posidonia oceanica seedlings (Herman and Sultan, 2016; Guerrero-Meseguer et al., 2017), and also increased their susceptibility to consumption by grazers (Hernán et al., 2016). Likewise, experiments with Z. japonica seedlings showed a thermal limit of 29°C above which seedlings die (Abe et al., 2009), similar to the thermal limit for P. oceanica seedlings. Seagrass seedling mortality at temperatures around 30°C are likely to constrain the extant distribution of seagrasses (e.g., Abe et al., 2009), and their capacity to accommodate to future, warmer regimes.
Elevated CO2 is believed to positively influence seagrasses, which are often CO2 limited (Koch et al., 2013). This also applies to seedlings, as P. oceanica seedlings grown under elevated CO2 improved photosynthetic performance, and developed larger carbon storage in belowground tissues, having thus more resources to tolerate and recover from stressors. However, elevated CO2 also favors filamentous algae, which can overgrow seagrass seedlings, leading to reduced growth (Burnell et al., 2014). Moreover, lower N content and increased sucrose levels in seedlings growing under high pCO2 lead to higher herbivory pressure (Hernán et al., 2016).
B3 Biotic Interactions—Increased Grazing Pressure
Indirect effects of rising temperatures are often mediated by biotic interactions. For macrophytes, increased grazing pressure is likely the most important indirect effect of the tropicalization of temperate seas as herbivores are progressively moving poleward (Vergés et al., 2014; Hyndes et al., 2016). Herbivore-induced shifts from productive kelp forests to turf substrate or barren grounds are already documented in the Mediterranean, Japan, and Australia (Vergés et al., 2014). For example, in a tropical-temperate transition zone in Eastern Australia, tropical herbivorous fishes contributed to the deforestation of kelp communities within ten years as sea surface temperature increased by 0.6°C (Vergés et al., 2016).
With rising temperatures, detrital-based food webs, supported by temperate seagrass ecosystems, are likely to turn into ones that are based on the direct consumption of seagrass (Lal et al., 2010; Kelkar et al., 2013; Hyndes et al., 2016). While few temperate species use seagrass as primary food source (Heck and Valentine, 2006), poleward-extending herbivorous fishes and macrograzers such as dugongs and marine turtles will likely have highest impact on temperate seagrass meadows in the winter months, when low light levels limit growth (Hyndes et al., 2016).
In addition to the northward shift of tropical herbivores, temperate calcified herbivores are becoming more abundant with rising temperatures (Harley et al., 2012), and further increase grazing pressure on temperate macroalgae. For example, the loss in cover of the fucoid macroalga Ascophyllum nodosum in regions of Northern Ireland was accompanied by increases in limpet densities due to a series of mild winters since the 1980s (Davies et al., 2007). While even small herbivores, such as limpets, can graze down mature A. nodosum monocultures (Lorenzen, 2007), the species that are most susceptible to increased grazing are those with small generation times, as herbivores can prevent the nearly annual germling recruitment that these macroalgae depend on (Jenkins et al., 2005; Coleman et al., 2006; Hawkins et al., 2008; Bennett et al., 2015; Franco et al., 2015). However, how the contrasting effect of rising temperatures and ocean acidification on calcified herbivores will affect canopy-forming macroalgae, is yet poorly understood (reviewed in Harley et al., 2012).
While the impact of increased herbivory on temperate macrophytes will certainly be strong, it may be mitigated by epigenetic or microbiome shifts that affect defense mechanisms. Initial studies in terrestrial plants demonstrate that epigenetic variation can influence plant-herbivore interactions across generations (Herrera and Bazaga, 2011; Holeski et al., 2012; Latzel et al., 2012; Rasmann et al., 2012). Moreover, beneficial microbes have been shown to enhance defense against insect herbivores (Pangesti et al., 2013; Pieterse et al., 2014). In marine macrophytes, the potential for non-genetic rapid adaptation to increased herbivory is entirely unknown. Initial studies may focus on the correlation between epigenetic or microbiome variation and variation in compensatory growth (Vergés et al., 2008) or in defense chemicals, such as phlorotannins and phenolic compounds (Hay and Fenical, 1988; Arnold and Targett, 2002; Vergés et al., 2007).
C Integrative Modeling–Understanding the Past and Modeling the Future
These plastic and evolutionary dimensions of seagrass and macroalgae performance under climate change are difficult to synthesize into a unifying concept/vision. The development of modeling tools able to incorporate relevant processes and parameters appears as a powerful tool to provide a holistic scenario and, thus, project climate change effects accounting for the diverse dimensions involved in seagrass and macroalgae production.
Nonetheless, numerical models of marine primary producers are still scarce in the scientific literature. Some of the first published models targeted phytoplankton productivity (Falkowski and Wirick, 1981); during the 90's with the global onset of coastal eutrophication, models of opportunistic macroalgal species growth (mostly Ulva spp.) became rather popular (Bendoricchio et al., 1993; Fong et al., 1997; Solidoro et al., 1997; Martins and Marques, 2002). More recently, seagrass and kelp models are getting prominent due to the growing recognition of their important ecosystem services and the increasing need to preserve them (Elkalay et al., 2003; Plus et al., 2003; Koch et al., 2007; Ortiz, 2008; Downie et al., 2013; Young et al., 2015; Franco et al., 2018). Thus, from the analysis of published literature, it is possible to identify two fundamentally different but complementary modeling approaches that have been applied to study primary producers: species distribution models (SDMs) and productivity models.
Species distribution models, also called “Ecological Niche Models” (ENMs) (Guisan and Zimmermann, 2000; Kelly et al., 2001; Murphy and Lovett-Doust, 2007; Elith and Leathwick, 2009; Kearney et al., 2009), have been applied to seagrasses and macroalgae, aiming to predict species distribution and habitat suitability (Downie et al., 2013; Jueterbock et al., 2013; Assis et al., 2016b, 2017a,b; Neiva et al., 2016) or to unveil past species range shifts (Assis et al., 2016a,b; Neiva et al., 2016; Chefaoui and Serrão, 2017; Chefaoui et al., 2017). In general, results from SDM's of temperate marine macroalgae predict that rising temperatures will cause massive die-offs along warm-temperate distribution limits and disclose new thermally suitable habitat in the Arctic (Müller et al., 2009; Jueterbock et al., 2013; Assis et al., 2017a). Besides projecting the distribution of species abundance, SDM's can also identify the main drivers associated with the presence/absence of seagrasses and macroalgae across coastal and marine areas (Chefaoui and Serrão, 2017), genetic diversity hotspots of long-term persistence, adaptive potential for several species (Bellard et al., 2012; Beaumont et al., 2016; Chefaoui and Serrão, 2017; Chefaoui et al., 2017), estimate seagrass colonization area or the probability of successful restoration (Kelly et al., 2001).
For marine macrophytes, both kelp and seagrasses, temperature was generally identified as the most important range-limiting factor and, consequently, ocean warming as the most severe threat among other global climate drivers (Downie et al., 2013; Jueterbock et al., 2013; Assis et al., 2016b, 2017a,b; Neiva et al., 2016; Chefaoui et al., 2017). Nevertheless, macrophytes can be exposed to extreme temperatures along their distribution ranges, especially in the intertidal zone where desiccation can prevent cellular stress and allow persistence under temperatures beyond their thermal tolerances (Mota et al., 2015). Thus, site-specific and species-specific relevant processes must be accounted for in order to build accurate SDM's of marine macrophytes and macroalgae (Assis et al., 2016b; Neiva et al., 2016). These models can give a first rough approximation of future distributions but they often do not take biological aspects or the eco-evolutionary responding potential of the focal species into account (but see Assis et al., 2016b).
Productivity models aim at describing the growth of algae and plants in a specific system, either through static (Eilers and Peeters, 1988) or dynamic (time-dependent) (Duarte and Ferreira, 1997) approaches, whereby the underlying mathematics is either empirical (Adams et al., 2017) or mechanistic (for a review see Macedo and Duarte, 2006). A major complication of the mechanistic approach is that its implementation requires extensive knowledge on the physiology and life history of the modeled species. Indeed, these models can even account for different size-classes or population groups (Duarte and Ferreira, 1997; Martins et al., 2007), which will add realism to the model, though it can also increase model instability due to the high number of variables (Duarte and Ferreira, 1997). Dealing with this requires application of the parsimony rule to model complexity vs. data quality throughout the entire modeling process (Jørgensen, 1994).
Productivity models have provided sensible descriptions of standing stock variations of marine primary producers, assessed the sensitivity of parameters and projected variations for scenarios of climatic changes and other stressors (Duarte and Ferreira, 1997; Fong et al., 1997; Elkalay et al., 2003; Lirman and Cropper, 2003; Biber et al., 2004; Martins et al., 2007; Couto et al., 2014). Nonetheless, there is a generalized consensus that marine primary production models should aim for increasing accuracy of parameters, spatial discrimination and uncertainty quantification (Coffaro et al., 1997; Lirman and Cropper, 2003; Biber et al., 2004; Laufkötter et al., 2015).
Multi-model approaches have the potential to compensate the weaknesses and bias associated to a single type of model and can provide richer information regarding the system. The ultimate goal is to integrate information on species' plasticity, adaptability, dispersal potential, and biotic interactions into a single forecasting approach (Moore et al., 2007; Lavergne et al., 2010). Although the need for an integrative approach is well recognized (Guisan and Thuiller, 2005; Guisan et al., 2006; Lavergne et al., 2010; Sinclair et al., 2010; Franco et al., 2018), implementation is largely unexplored and lacks consensus. Based on high-quality data retrieved from comprehensive environmental datasets (van Vuuren et al., 2011; Taylor et al., 2012; Tyberghein et al., 2012; Assis et al., 2017a) and empirical approaches (Franco et al., 2018), we propose a stepwise multi-model strategy that combines correlative SDMs with mechanistic productivity models into an integrative forecasting tool aimed at understanding changes at the ecosystem level. This multi-model approach comprises:
- Building SDMs to identify key environmental drivers for the distribution of seagrass/macroalgae, to predict sensitive areas where the species are threatened with extinction and robust areas for restoration;
- Developing productivity models for the dominant species incorporating species- and site-specific parameters identified in the previous step; these models should account for the spatial variability of the modeled area (e.g., depth, type of sediment, wave exposure) and the ecotypic differentiation of the focal species in terms of plastic and adaptive potential.
This stepwise modeling approach would provide dynamic and spatial explicit simulations of seagrass and macroalgal ecosystems coupled to an accurate evaluation of uncertainty at each stage of the modeling process (Larson et al., 2004). Implementing such a modeling framework is a challenging task that, nonetheless, is worthwhile trying as it will improve the power to predict range shifts and assessing species extinction risks under climate change. This is of paramount importance for an effective management of marine primary producer habitats and derived ecosystem services.
Final Remarks
Seagrasses and macroalgae are in multiple ways at stake under the ongoing climatic changes. Physiological responses and the survivability of early life stages (both seeds and spores) are intrinsically connected to their genetic and epigenetic characteristics. Additionally, environmental changes affect the symbiotic relation between microbiome communities and their hosts. Biochemical changes in macrophytes can have severe impacts on trophic levels feeding on seagrass derived organic matter, including reduced energy transfer due to reduced carbon fixation, but also severe reduction of essential fatty acid production. In order to predict with a more holistic understanding the constrains to which these important foundation species will be subjected in the near future, the concept of niche stability in conventional modeling approaches should be widened by coupling physiological and ecological insights in primary productivity models and ecological niche models. Ideally, research on seagrasses and macroalgae should be multi-disciplinary, integrating genetic, epigenetic, and microbiome levels of intra-specific variation and ecotypic differentiation for a comprehensive understanding of phenotypic variation and more realistic scenarios of change that are essential for mitigation and conservation purposes (Figure 1).
Author Contributions
IM and AJ wrote the section focusing modeling approaches. RR, AM, and BD prepared the section regarding the physiological impacts of climate change. MR and CD worked on the section regarding the early life stages. The biogeographical gradients section was prepared GP and AJ. The epigenetics section was developed by TR and AJ. ES and AE focused on the microbiome section. BD gathered all the sections into this unified manuscript, supervised by all authors and specially by IC, JM, AJ, ES, and CD.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank to the Fundação para a Ciência e Tecnologia (FCT) for funding the research in the Marine and Environmental Sciences Centre (MARE) throughout the project UID/MAR/04292/2013, the Biosystems and Integrative Sciences Institute (BioISI) throughout the project UID/MULTI/04046/2013, the Centre of Marine Sciences (CCMAR) throughout the project UID/Multi/04326/2013 and the Interdisciplinary Centre of Marine and Environmental Research (CIIMAR) throughout the project UID/Multi/04423/2013. BD investigation was supported by FCT throughout a Postdoctoral grant (SFRH/BPD/115162/2016). ES and GP thank the Pew Foundation (USA), the Portuguese FCT through MARFOR (Biodiversa/0004/2015) and a postdoctoral fellowship (SFRH/PBD/107878/ 2015) to AE. AJ is supported by the Norwegian Research Council (Havkyst project 243916). IM is partially supported by the European Regional Development Fund (ERDF), in the framework of the program PT2020. The authors would also like to thank to the Mar 2020 program through the VALPRAD project (16-01-04-FMP-0007). We acknowledge the two reviewers for their comments and suggestions that helped to improve the structure and quality of this review.
References
Abe, M., Yokota, K., Kurashima, A., and Maegawa, M. (2009). High water temperature tolerance in photosynthetic activity of Zostera japonica Ascherson & Graebner seedlings from Ago Bay, Mie Prefecture, central Japan. Fish. Sci. 75, 1117–1123. doi: 10.1007/s12562-009-0141-x
Adams, M. P., Collier, C. J., Uthicke, S., Ow, Y. X., Langlois, L., and O'Brien, K. R. (2017). Model fit versus biological relevance: evaluating photosynthesis-temperature models for three tropical seagrass species. Sci. Rep. 7:39930. doi: 10.1038/srep39930
Adusumalli, S., Omar, M. F. M., Soong, R., and Benoukraf, T. (2014). Methodological aspects of whole-genome bisulfite sequencing analysis. Brief. Bioinform. 16, 369–379. doi: 10.1093/bib/bbu016
Aires, T., Marbà, N., Cunha, R. L., Kendrick, G. A., Walker, D. I., Serrão, E. A., et al. (2011). Evolutionary history of the seagrass genus Posidonia. Mar. Ecol. Prog. Ser. 421, 117–130. doi: 10.3354/meps08879
Aires, T., Serrão, E. A., and Engelen, A. H. (2016). Host and environmental specificity in bacterial communities associated to two highly invasive marine species (genus Asparagopsis). Front. Microbiol. 7:559. doi: 10.3389/fmicb.2016.00559
Aires, T., Serrão, E. A., Kendrick, G., Duarte, C. M., and Arnaud-haond, S. (2013). Invasion is a community affair : clandestine followers in the bacterial community associated to Green Algae, Caulerpa racemosa, Track the Invasion Source. PLoS ONE 8:e68429. doi: 10.1371/journal.pone.0068429
Alberto, F., Massa, S., Manent, P., Diaz-Almela, E., Arnaud-Haond, S., Duarte, C. M., et al. (2008). Genetic differentiation and secondary contact zone in the seagrass Cymodocea nodosa across the Mediterranean-Atlantic transition region. J. Biogeogr. 35, 1279–1294. doi: 10.1111/j.1365-2699.2007.01876.x
Alberto, F., Raimondi, P. T., Reed, D. C., Watson, J. R., Siegel, D. A., Mitarai, S., et al. (2011). Isolation by oceanographic distance explains genetic structure for Macrocystis pyrifera in the Santa Barbara Channel. Mol. Ecol. 20, 2543–2554. doi: 10.1111/j.1365-294X.2011.05117.x
Alexander, L. V., Zhang, X., Peterson, T. C., Caesar, J., Gleason, B., Klein Tank, A. M. G., et al. (2006). Global observed changes in daily climate extremes of temperature and precipitation. J. Geophys. Res. Atmos. 111. doi: 10.1029/2005JD006290
Anastasiadi, D., Díaz, N., and Piferrer, F. (2017). Small ocean temperature increases elicit stage-dependent changes in DNA methylation and gene expression in a fish, the European sea bass. Sci. Rep. 7, 1–12. doi: 10.1038/s41598-017-10861-6
Araújo, R. M., Assis, J., Aguillar, R., Airoldi, L., Bárbara, I., Bartsch, I., et al. (2016). Status, trends and drivers of kelp forests in Europe: an expert assessment. Biodivers. Conserv. 25, 1319–1348. doi: 10.1007/s10531-016-1141-7
Arnaud-Haond, S., Aires, T., Candeias, R., Teixeira, S. J. L., Duarte, C. M., Valero, M., et al. (2017). Entangled fates of holobiont genomes during invasion : nested bacterial and host diversities in Caulerpa taxifolia. Mol. Ecol. 26, 2379–2391. doi: 10.1111/mec.14030
Arnaud-Haond, S., Duarte, C. M., Alberto, F., and Serrão, E. A. (2007a). Standardizing methods to address clonality in population studies. Mol. Ecol. 16, 5115–5139. doi: 10.1111/j.1365-294X.2007.03535.x
Arnaud-Haond, S., Duarte, C. M., Diaz-Almela, E., Marbà, N., Sintes, T., and Serrão, E. A. (2012). Implications of extreme life span in clonal organisms: millenary clones in meadows of the threatened seagrass posidonia oceanica. PLoS ONE 7:e30454. doi: 10.1371/journal.pone.0030454
Arnaud-Haond, S., Migliaccio, M., Diaz-Almela, E., Teixeira, S., Van De Vliet, M. S., Alberto, F., et al. (2007b). Vicariance patterns in the Mediterranean Sea: East-west cleavage and low dispersal in the endemic seagrass Posidonia oceanica. J. Biogeogr. 34, 963–976. doi: 10.1111/j.1365-2699.2006.01671.x
Arnold, T. M., and Targett, N. M. (2002). Marine tannins: the importance of a mechanistic framework for predicting ecological roles. J. Chem. Ecol. 28, 1919–1934. doi: 10.1023/A:1020737609151
Assis, J., Araújo, M. B., and Serrão, E. A. (2017a). Projected climate changes threaten ancient refugia of kelp forests in the North Atlantic. Glob. Chang. Biol. 24, e55–e66 doi: 10.1111/gcb.13818
Assis, J., Berecibar, E., Claro, B., Alberto, F., Reed, D., Raimondi, P., et al. (2017b). Major shifts at the range edge of marine forests: the combined effects of climate changes and limited dispersal. Sci. Rep. 7:44348. doi: 10.1038/srep44348
Assis, J., Coelho, N. C., Lamy, T., Valero, M., Alberto, F., and Serrão, E. Á. (2016a). Deep reefs are climatic refugia for genetic diversity of marine forests. J. Biogeogr. 43, 833–844. doi: 10.1111/jbi.12677
Assis, J., Lucas, A. V., Bárbara, I., and Serrão, E. Á. (2016b). Future climate change is predicted to shift long-term persistence zones in the cold-temperate kelp Laminaria hyperborea. Mar. Environ. Res. 113, 174–182. doi: 10.1016/j.marenvres.2015.11.005
Badri, D. V., Zolla, G., Bakker, M. G., Manter, D. K., and Vivanco, J. M. (2013). Potential impact of soil microbiomes on the leaf metabolome and on herbivore feeding behavior. New Phytol. 198, 264–273. doi: 10.1111/nph.12124
Balestri, E., and Cinelli, F. (2003). Sexual reproductive success in Posidonia oceanica. Aquat. Bot. 75, 21–32. doi: 10.1016/S0304-3770(02)00151-1
Barriopedro, D., Fischer, E. M., Luterbacher, J., Trigo, R. M., and García-Herrera, R. (2011). The hot summer of 2010: redrawing the temperature record map of Europe. Science 332, 220–224. doi: 10.1126/science.1201224
Bartsch, I., Vogt, J., Pehlke, C., and Hanelt, D. (2013). Prevailing sea surface temperatures inhibit summer reproduction of the kelp Laminaria digitata at Helgoland (North Sea). J. Phycol. 49, 1061–1073. doi: 10.1111/jpy.12125
Bartsch, I., Wiencke, C., Bischof, K., Buchholz, C. M., Buck, B. H., Eggert, A., et al. (2008). The genus Laminaria sensu lato: recent insights and developments. Eur. J. Phycol. 43, 1–86. doi: 10.1080/09670260701711376
Beardall, J., Beer, S., and Raven, J. A. (1998). Biodiversity of marine plants in an era of climate change: some predictions based on physiological performance. Bot. Mar. 41, 113–123. doi: 10.1515/botm.1998.41.1-6.113
Beaumont, L. J., Graham, E., Duursma, D. E., Wilson, P. D., Cabrelli, A., Baumgartner, J. B., et al. (2016). Which species distribution models are more (or less) likely to project broad-scale, climate-induced shifts in species ranges? Ecol. Model. 342, 135–146. doi: 10.1016/j.ecolmodel.2016.10.004
Bellard, C., Bertelsmeier, C., Leadley, P., Thuiller, W., and Courchamp, F. (2012). Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365–377. doi: 10.1111/j.1461-0248.2011.01736.x
Bendoricchio, G., Coffaro, G., and Di Luzio, M. (1993). Modelling the photosynthetic efficiency for Ulva rigida growth. Ecol. Model. 67, 221–232. doi: 10.1016/0304-3800(93)90006-E
Bengtsson, M. M., and Øvreås, L. (2010). Planctomycetes dominate biofilms on surfaces of the kelp Laminaria hyperborea. BMC Microbiol. 10:261. doi: 10.1186/1471-2180-10-261
Bengtsson, M. M., Sjøtun, K., and Øvreås, L. (2010). Seasonal dynamics of bacterial biofilms on the kelp Laminaria hyperborea. Aquat. Microb. Ecol. 60, 71–83. doi: 10.3354/ame01409
Bengtsson, M. M., Sjøtun, K., Lanzén, A., and Øvreås, L. (2012). Bacterial diversity in relation to secondary production and succession on surfaces of the kelp Laminaria hyperborea. ISME J. 6, 2188–2198. doi: 10.1038/ismej.2012.67
Beniston, M., and Stephenson, D. B. (2004). ScienceDirect.com - Global and Planetary Change - Extreme climatic events and their evolution under changing climatic conditions. Glob. Planet. Change 44, 1–9. doi: 10.1016/j.gloplacha.2004.06.001
Bennett, S., Wernberg, T., Harvey, E. S., Santana-Garcon, J., and Saunders, B. J. (2015). Tropical herbivores provide resilience to a climate-mediated phase shift on temperate reefs. Ecol. Lett. 18, 714–723. doi: 10.1111/ele.12450
Berge, J., Renaud, P. E., Darnis, G., Cottier, F., Last, K., Gabrielsen, T. M., et al. (2015). In the dark: a review of ecosystem processes during the Arctic polar night. Prog. Oceanogr. 139, 258–271. doi: 10.1016/j.pocean.2015.08.005
Berger, S. L., Kouzarides, T., Shiekhattar, R., and Shilatifard, A. (2009). An operational definition of epigenetics. Genes Dev. 23, 781–783. doi: 10.1101/gad.1787609
Biber, P. D., Harwell, M. A., and Cropper, W. P. (2004). Modeling the dynamics of three functional groups of macroalgae in tropical seagrass habitats. Ecol. Modell. 175, 25–54. doi: 10.1016/j.ecolmodel.2003.10.003
Biémont, C. (2010). A brief history of the status of transposable elements: from junk DNA to major players in evolution. Genetics 186, 1085–1093. doi: 10.1534/genetics.110.124180
Bijlsma, R., and Loeschcke, V. (2012). Genetic erosion impedes adaptive responses to stressful environments. Evol. Appl. 5, 117–129. doi: 10.1111/j.1752-4571.2011.00214.x
Bockelmann, A. C., Beining, K., and Reusch, T. B. H. (2012). Widespread occurrence of endophytic Labyrinthula spp. in northern European eelgrass Zostera marina beds. Mar. Ecol. Prog. Ser. 445, 109–116. doi: 10.3354/meps09398
Bockelmann, A. C., Tams, V., Ploog, J., Schubert, P. R., and Reusch, T. B. H. (2013). Quantitative PCR Reveals Strong Spatial and Temporal Variation of the Wasting Disease Pathogen, Labyrinthula zosterae in Northern European Eelgrass (Zostera marina) Beds. PLoS ONE 8:e62169. doi: 10.1371/journal.pone.0062169
Bolton, J. J. (2010). The biogeography of kelps (Laminariales, Phaeophyceae): a global analysis with new insights from recent advances in molecular phylogenetics. Helgol. Mar. Res. 64, 263–279. doi: 10.1007/s10152-010-0211-6
Bonasio, R. (2015). The expanding epigenetic landscape of non-model organisms. J. Exp. Biol. 218, 114–122. doi: 10.1242/jeb.110809
Bossdorf, O., Richards, C. L., and Pigliucci, M. (2008). Epigenetics for ecologists. Ecol. Lett. 11, 106–115. doi: 10.1111/j.1461-0248.2007.01130.x
Bourne, D. G., Morrow, K. M., and Webster, N. S. (2016). Insights into the Coral Microbiome: underpinning the Health and Resilience of Reef Ecosystems. Annu. Rev. Microbiol. 70, 317–340. doi: 10.1146/annurev-micro-102215-095440
Brakel, J., Reusch, T. B. H., and Bockelmann, A. C. (2017). Moderate virulence caused by the protist Labyrinthula zosterae in ecosystem foundation species Zostera marina under nutrient limitation. Mar. Ecol. Prog. Ser. 571, 97–108. doi: 10.3354/meps12104
Brakel, J., Werner, F. J., Tams, V., Reusch, T. B. H., and Bockelmann, A. C. (2014). Current European Labyrinthula zosterae are not virulent and modulate seagrass (Zostera marina) defense gene expression. PLoS ONE 9:e92448. doi: 10.1371/journal.pone.0092448
Brawley, S. H., and Johnson, L. E. (1991). Survival of fucoid embryos in the intertidal zone depends upon developmental stage and microhabitat. J. Phycol. 27, 179–186. doi: 10.1111/j.0022-3646.1991.00179.x
Brodie, J., Williamson, C. J., Smale, D. A., Kamenos, N. A., Mieszkowska, N., Santos, R., et al. (2014). The future of the northeast Atlantic benthic flora in a high CO2 world. Ecol. Evol. 4, 2787–2798. doi: 10.1002/ece3.1105
Burgess, J. G., Jordan, E. M., Bregu, M., Mearns-Spragg, A., and Boyd, K. G. (1999). Microbial antagonism: a neglected avenue of natural products research. J. Biotechnol. 70, 27–32.
Burnell, O. W., Russell, B. D., Irving, A. D., and Connell, S. D. (2014). Seagrass response to CO2 contingent on epiphytic algae : indirect effects can overwhelm direct effects. Oecologia 176, 871–882. doi: 10.1007/s00442-014-3054-z
Calder, P. C. (2015). Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 39, 18S–32S. doi: 10.1177/0148607115595980
Campbell, S. J., McKenzie, L. J., and Kerville, S. P. (2006). Photosynthetic responses of seven tropical seagrasses to elevated seawater temperature. J. Exp. Mar. Bio. Ecol. 330, 455–468. doi: 10.1016/j.jembe.2005.09.017
Casacuberta, E., and González, J. (2013). The impact of transposable elements in environmental adaptation. Mol. Ecol. 22, 1503–1517. doi: 10.1111/mec.12170
Chaparro, J. M., Badri, D. V., and Vivanco, J. M. (2014). Rhizosphere microbiome assemblage is affected by plant development. ISME J. 8, 790–803. doi: 10.1038/ismej.2013.196
Chefaoui, R. M., and Serrão, E. A. (2017). Accounting for uncertainty in predictions of a marine species: integrating population genetics to verify past distributions. Ecol. Model. 359, 229–239. doi: 10.1016/j.ecolmodel.2017.06.006
Chefaoui, R. M., Duarte, C. M., and Serrão, E. A. (2017). Palaeoclimatic conditions in the Mediterranean explain genetic diversity of Posidonia oceanica seagrass meadows. Sci. Rep. 7:2732. doi: 10.1038/s41598-017-03006-2
Chénais, B., Caruso, A., Hiard, S., and Casse, N. (2012). The impact of transposable elements on eukaryotic genomes: from genome size increase to genetic adaptation to stressful environments. Gene 509, 7–15. doi: 10.1016/j.gene.2012.07.042
Chung, I. K., Beardall, J., Mehta, S., Sahoo, D., and Stojkovic, S. (2011). Using marine macroalgae for carbon sequestration: a critical appraisal. J. Appl. Phycol. 23, 877–886. doi: 10.1007/s10811-010-9604-9
Clasen, J. L., and Shurin, J. B. (2015). Kelp forest size alters microbial community structure and function on Vancouver Island, Canada. Ecology 96, 862–872. doi: 10.1890/13-2147.1.sm
Cock, J. M., Coelho, S. M., Brownlee, C., and Taylor, A. R. (2010). The Ectocarpus genome sequence: insights into brown algal biology and the evolutionary diversity of the eukaryotes. New Phytol. 188, 1–4. doi: 10.1111/j.1469-8137.2010.03454.x
Coffaro, G., Bocci, M., and Bendoricchio, G. (1997). Application of structural dynamic approach to estimate space variability of primary producers in shallow marine water. Ecol. Modell. 102, 97–114. doi: 10.1016/S0304-3800(97)00097-5
Coleman, R. A., Underwood, A. J., Benedetti-Cecchi, L., Åberg, P., Arenas, F., Arrontes, J., et al. (2006). A continental scale evaluation of the role of limpet grazing on rocky shores. Oecologia 147, 556–564. doi: 10.1007/s00442-005-0296-9
Collier, C. J., Ow, Y. X., Langlois, L., Uthicke, S., Johansson, C. L., O'Brien, K. R., et al. (2017). Optimum temperatures for net primary productivity of three tropical Seagrass Species. Front. Plant Sci. 8:1446. doi: 10.3389/fpls.2017.01446
Costanza, R., Arge, R., Groot, R., De Farber, S., Grasso, M., Hannon, B., et al. (1997). The value of the world ' s ecosystem services and natural capital. Nature 387, 253–260.
Couto, T., Martins, I., Duarte, B., Caçador, I., and Marques, J. C. (2014). Modelling the effects of global temperature increase on the growth of salt marsh plants. Appl. Ecol. Environ. Res. 12, 753–764. doi: 10.15666/aeer/1203_753764
Coyer, J. A., Diekmann, O. E., Serrão, E. A., Procaccini, G., Milchakova, N., Pearson, G. A., et al. (2004). Population genetics of dwarf eelgrass Zostera noltii throughout its biogeographic range. Mar. Ecol. Prog. Ser. 281, 51–62. doi: 10.3354/meps281051
Coyer, J. A., Hoarau, G., Kuo, J., Tronholm, A., Veldsink, J., and Olsen, J. L. (2013). Phylogeny and temporal divergence of the seagrass family Zosteraceae using one nuclear and three chloroplast loci. Syst. Biodivers. 11, 271–284. doi: 10.1080/14772000.2013.821187
Croft, M. T., Lawrence, A. D., Raux-Deery, E., Warren, M. J., and Smith, A. G. (2005). Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438, 90–93. doi: 10.1038/nature04056
Crump, B., Wojahn, J., Tomas, F., and Mueller, R. (2018). Metatranscriptomics and amplicon sequencing reveal mutualisms in seagrass microbiomes. Front. Microbiol. 9:388. doi: 10.3389/fmicb.2018.00388
Cúcio, C., Engelen, A. H., Costa, R., and Muyzer, G. (2016). Rhizosphere microbiomes of European + seagrasses are selected by the plant, but are not species specific. Front. Microbiol. 7:440. doi: 10.3389/fmicb.2016.00440
Darling, E. S., and Côté, I. M. (2008). Quantifying the evidence for ecological synergies. Ecol. Lett. 11, 1278–1286. doi: 10.1111/j.1461-0248.2008.01243.x
Davies, A. J., Johnson, M. P., and Maggs, C. A. (2007). Limpet grazing and loss of Ascophyllum nodosum canopies on decadal time scales. Mar. Ecol. Prog. Ser. 339, 131–141. doi: 10.3354/meps339131
Davison, I. R. (1991). Environmental effects on algal photosynthesis: temperature. J. Phycol. 27, 2–8.
De Boer, W. F. (2007). Seagrass-sediment interactions, positive feedbacks and critical thresholds for occurrence: a review. Hydrobiologia 591, 5–24. doi: 10.1007/s10750-007-0780-9
De Meester, L., Gómez, A., Okamura, B., and Schwenk, K. (2002). The Monopolization Hypothesis and the dispersal-gene flow paradox in aquatic organisms. Acta Oecol. 23, 121–135. doi: 10.1016/S1146-609X(02)01145-1
Delebecq, G., Davoult, D., Janquin, M. A., Oppliger, L. V., Menu, D., Dauvin, J. C., et al. (2016). Photosynthetic response to light and temperature in Laminaria digitata gametophytes from two French populations. Eur. J. Phycol. 51, 71–82. doi: 10.1080/09670262.2015.1104556
Diaz-Almela, E., Marbà, N., and Duarte, C. M. (2007). Consequences of Mediterranean warming events in seagrass (Posidonia oceanica) flowering records. Glob. Chang. Biol. 13, 224–235. doi: 10.1111/j.1365-2486.2006.01260.x
Diekmann, O. E., and Serrão, E. A. (2012). Range-edge genetic diversity: locally poor extant southern patches maintain a regionally diverse hotspot in the seagrass Zostera marina. Mol. Ecol. 21, 1647–1657. doi: 10.1111/j.1365-294X.2012.05500.x
Diekmann, O. E., Coyer, J. A., Ferreira, J., Olsen, J. L., Stam, W. T., Pearson, G. A., et al. (2005). Population genetics of Zostera noltii along the west Iberian coast: consequences of small population size, habitat discontinuity and near-shore currents. Mar. Ecol. Prog. Ser. 290, 89–96. doi: 10.3354/meps290089
Dimitrieva, G. Y., Crawford, R. L., and Yüksel, G. Ü. (2006). The nature of plant growth-promoting effects of a pseudoalteromonad associated with the marine algae Laminaria japonica and linked to catalase excretion. J. Appl. Microbiol. 100, 1159–1169. doi: 10.1111/j.1365-2672.2006.02831.x
Dittami, S. M., Duboscq-Bidot, L., Perennou, M., Gobet, A., Corre, E., Boyen, C., et al. (2016). Host–microbe interactions as a driver of acclimation to salinity gradients in brown algal cultures. ISME J. 10, 51–63. doi: 10.1038/ismej.2015.104
Domingues, C. M., Church, J. A., White, N. J., Gleckler, P. J., Wijffels, S. E., Barker, P. M., et al. (2008). Improved estimates of upper-ocean warming and multi-decadal sea-level rise. Nature 453, 1090–1093. doi: 10.1038/nature07080
Doney, S. C., Fabry, V. J., Feely, R. A., and Kleypas, J. A. (2009). Ocean acidification: the other CO2 Problem 169–194. doi: 10.1146/annurev.marine.010908.163834
Doney, S. C., Ruckelshaus, M., Emmett Duffy, J., Barry, J. P., Chan, F., English, C. A., et al. (2012). Climate change impacts on marine ecosystems. Ann. Rev. Mar. Sci. 4, 11–37. doi: 10.1146/annurev-marine-041911-111611
Douglas, A. E., and Werren, J. H. (2016). Holes in the hologenome: why host-microbe symbioses are not holobionts. mBio 7:e02099-15. doi: 10.1128/mBio.02099-15
Douhovnikoff, V., and Dodd, R. S. (2014). Epigenetics: a potential mechanism for clonal plant success. Plant Ecol. 216, 227–233. doi: 10.1007/s11258-014-0430-z
Downie, A.-L., von Numers, M., and Boström, C. (2013). Influence of model selection on the predicted distribution of the seagrass Zostera marina. Estuar. Coast. Shelf Sci. 121–122, 8–19. doi: 10.1016/j.ecss.2012.12.020
Duarte, B., and Caçador, I. (2012). Particulate metal distribution in Tagus estuary (Portugal) during a flood episode. Mar. Pollut. Bull. 64, 2109–2116. doi: 10.1016/j.marpolbul.2012.07.016
Duarte, B., Goessling, J. W., Marques, J. C., and Caçador, I. (2015a). Ecophysiological constraints of Aster tripolium under extreme thermal events impacts: merging biophysical, biochemical and genetic insights. Plant Physiol. Biochem. 97, 217–228. doi: 10.1016/j.plaphy.2015.10.015
Duarte, B., Marques, J. C., and Caçador, I. (2016). Ecophysiological response of native and invasive Spartina species to extreme temperature events in Mediterranean marshes. Biol. Invasions 18, 2189–2205. doi: 10.1007/s10530-015-0958-4
Duarte, B., Pedro, S., Marques, J. C., Adão, H., and Caçador, I. (2017a). Zostera noltii development probing using chlorophyll a transient analysis (JIP-test) under field conditions: integrating physiological insights into a photochemical stress index. Ecol. Indic. 76, 219–229. doi: 10.1016/j.ecolind.2017.01.023
Duarte, B., Santos, D., Marques, J. C., and Caçador, I. (2015b). Impact of heat and cold events on the energetic metabolism of the C3 halophyte Halimione portulacoides. Estuar. Coast. Shelf Sci. 167, 166–177. doi: 10.1016/j.ecss.2015.10.003
Duarte, B., Vaz, N., Valentim, J. M., Dias, J. M., Silva, H., Marques, J. C., et al. (2017b). Revisiting the outwelling hypothesis: modelling salt marsh detrital metal exports under extreme climatic events. Mar. Chem. 191, 24–33. doi: 10.1016/j.marchem.2016.12.002
Duarte, C. M., Kennedy, H., Marbà, N., and Hendriks, I. (2013). Assessing the capacity of seagrass meadows for carbon burial: current limitations and future strategies. Ocean Coast. Manag. 83, 32–38. doi: 10.1016/j.ocecoaman.2011.09.001
Duarte, P., and Ferreira, J. G. (1997). A model for the simulation of macroalgal population dynamics and productivity. Ecol. Model. 98, 199–214. doi: 10.1016/S0304-3800(96)01915-1
Dubin, M. J., Zhang, P., Meng, D., Remigereau, M. S., Osborne, E. J., Casale, F. P., et al. (2015). DNA methylation in Arabidopsis has a genetic basis and shows evidence of local adaptation. Elife 4, 1–23. doi: 10.7554/eLife.05255
Egan, S., Fernandes, N. D., Kumar, V., Gardiner, M., and Thomas, T. (2014). Bacterial pathogens, virulence mechanism and host defence in marine macroalgae. Environ. Microbiol. 16, 925–938. doi: 10.1111/1462-2920.12288
Egan, S., Harder, T., Burke, C., Steinberg, P., Kjelleberg, S., and Thomas, T. (2013). The seaweed holobiont: understanding seaweed-bacteria interactions. FEMS Microbiol. Rev. 37, 462–476. doi: 10.1111/1574-6976.12011
Egan, S., Thomas, T., Holmström, C., and Kjelleberg, S. (2000). Phylogenetic relationship and antifouling activity of bacterial epiphytes from the marine alga Ulva lactuca. Environ. Microbiol. 2, 343–347. doi: 10.1046/j.1462-2920.2000.00107.x
Ehlers, A., Worm, B., and Reusch, T. B. H. (2008). Importance of genetic diversity in eelgrass Zostera marina for its resilience to global warming. Mar. Ecol. Prog. Ser. 355, 1–7. doi: 10.3354/meps07369
Eilers, P. H. C., and Peeters, J. C. H. (1988). A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Modell. 42, 199–215. doi: 10.1016/0304-3800(88)90057-9
Elith, J., and Leathwick, J. R. (2009). species distribution models: ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 40, 677–697. doi: 10.1146/annurev.ecolsys.110308.120159
Elkalay, K., Frangoulis, C., Skliris, N., Goffart, A., Gobert, S., Lepoint, G., et al. (2003). A model of the seasonal dynamics of biomass and production of the seagrass Posidonia oceanica in the Bay of Calvi (Northwestern Mediterranean). Ecol. Modell. 167, 1–18. doi: 10.1016/S0304-3800(03)00074-7
Falkowski, P. G., and Wirick, C. D. (1981). A simulation model of the effects of vertical mixing on primary productivity. Mar. Biol. 65, 69–75. doi: 10.1007/BF00397069
Feijão, E., Gameiro, C., Franzitta, M., Duarte, B., Caçador, I., Cabrita, M. T., et al. (in press). Heat wave impacts on the model diatom Phaeodactylum tricornutum: searching for photochemical fatty acid biomarkers of thermal stress. Ecol. Indic. doi: 10.1016/j.ecolind.2017.07.058
Feng, S., Jacobsen, S. E., and Reik, W. (2010). Epigenetic reprogramming in plant and animal development. Science 330, 622–627. doi: 10.1126/science.1190614.Epigenetic
Field, C., Barros, V., Stocker, T., and Dahe, Q. (eds.). (2012). Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation: Special Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Fong, P., Jacobson, M. E., Mescher, M. C., Lirman, D., and Harwell, M. C. (1997). Investigating the management potential of a seagrass model through sensitivity analysis and experiments. Ecol. Appl. 7, 300–315.
Fourqurean, J. W., and Zieman, J. C. (1991). Photosynthesis, respiration and whole plant carbon budget of the seagrass Thalassia testudinum. Mar. Ecol. Prog. Ser. 69, 161–170. doi: 10.3354/meps069161
Foust, C. M., Preite, V., Schrey, A. W., Alvarez, M., Robertson, M. H., Verhoeven, K. J. F., et al. (2016). Genetic and epigenetic differences associated with environmental gradients in replicate populations of two salt marsh perennials. Mol. Ecol. 25, 1639–1652. doi: 10.1111/mec.13522
Franco, J. N., Tuya, F., Bertocci, I., Rodríguez, L., Martínez, B., Sousa-Pinto, I., et al. (2018). The “golden kelp” Laminaria ochroleuca under global change: integrating multiple eco-physiological responses with species distribution models. J. Ecol. 106, 47–58. doi: 10.1111/1365-2745.12810
Franco, J. N., Wernberg, T., Bertocci, I., Duarte, P., Jacinto, D., Vasco-Rodrigues, N., et al. (2015). Herbivory drives kelp recruits into “hiding” in a warm ocean climate. Mar. Ecol. Prog. Ser. 536, 1–9. doi: 10.3354/meps11445
Fraser, M. W., Kendrick, G. A., Statton, J., Hovey, R. K., Zavala-Perez, A., and Walker, D. I. (2014). Extreme climate events lower resilience of foundation seagrass at edge of biogeographical range. J. Ecol. 102, 1528–1536. doi: 10.1111/1365-2745.12300
Fuschino, J. R., Guschina, I. A., Dobson, G., Yan, N. D., Harwood, J. L., and Arts, M. T. (2011). Rising water temperatures alter lipid dynamics and reduce n-3 essential fatty acid concentrations in Scenedesmus obliquus (chlorophyta). J. Phycol. 47, 763–774. doi: 10.1111/j.1529-8817.2011.01024.x
Gaitán-Espitia, J. D., Hancock, J. R., Padilla-Gamiño, J. L., Rivest, E. B., Blanchette, C. A., Reed, D. C., et al. (2014). Interactive effects of elevated temperature and pCO2 on early-life-history stages of the giant kelp Macrocystis pyrifera. J. Exp. Mar. Bio. Ecol. 457, 51–58. doi: 10.1016/j.jembe.2014.03.018
Gameiro, C., Utkin, A. B., Cartaxana, P., da Silva, J. M., and Matos, A. R. (2016). The use of laser induced chlorophyll fluorescence (LIF) as a fast and non-destructive method to investigate water deficit in Arabidopsis. Agric. Water Manag. 164, 127–136. doi: 10.1016/j.agwat.2015.09.008
Garcias-Bonet, N., Marbà, N., Holmer, M., and Duarte, C. M. (2008). Effects of sediment sulfides on seagrass Posidonia oceanica meristematic activity. Mar. Ecol. Prog. Ser. 372, 1–6. doi: 10.3354/meps07714
Garrabou, J., Coma, R., Bensoussan, N., Chevaldonné, P., Cigliano, M., Diaz, D., et al. (2009). A new large scale mass mortality event in the NW Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob. Chang. Biol. 15, 1090–1103. doi: 10.1111/j.1365-2486.2008.01823x
Gladyshev, M. I., Sushchik, N. N., and Makhutova, O. N. (2013). Production of EPA and DHA in aquatic ecosystems and their transfer to the land. Prostaglandins Other Lipid Mediat. 107, 117–126. doi: 10.1016/j.prostaglandins.2013.03.002
Goecke, F., Labes, A., Wiese, J., and Imhoff, J. F. (2010). Review chemical interactions between Marine macroalgae and bacteria. Mar. Ecol. Prog. Ser. 409, 267–300. doi: 10.3354/meps08607
González, J., Karasov, T. L., Messer, P. W., and Petrov, D. A. (2010). Genome-wide patterns of adaptation to temperate environments associated with transposable elements in Drosophila. PLoS Genet. 6:e1000905. doi: 10.1371/journal.pgen.1000905
Grueneberg, J., Engelen, A. H., Costa, R., and Wichard, T. (2016). Macroalgal morphogenesis induced by waterborne compounds and bacteria in coastal seawater. PLoS ONE 11:146307. doi: 10.1371/journal.pone.0146307
Gu, H., Smith, Z. D., Bock, C., Boyle, P., Gnirke, A., and Meissner, A. (2011). Preparation of reduced representation bisulfite sequencing libraries for genome-scale DNA methylation profiling. Nat. Protoc. 6, 468–481. doi: 10.1038/nprot.2010.190
Guerrero-Meseguer, L., Marín, A., and Sanz-l, C. (2017). Future heat waves due to climate change threaten the survival of Posidonia oceanica seedlings. Environ. Pollut. 230, 40–45. doi: 10.1016/j.envpol.2017.06.039
Gugger, P. F., Fitz-Gibbon, S., Pellegrini, M., and Sork, V. L. (2016). Species-wide patterns of DNA methylation variation in Quercus lobata and their association with climate gradients. Mol. Ecol. 25, 1665–1680. doi: 10.1111/mec.13563
Guisan, A., and Thuiller, W. (2005). Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 8, 993–1009. doi: 10.1111/j.1461-0248.2005.00792.x
Guisan, A., and Zimmermann, N. E. (2000). Predictive habitat distribution models in ecology. Ecol. Modell. 135, 147–186. doi: 10.1016/S0304-3800(00)00354-9
Guisan, A., Lehmann, A., Ferrier, S., Austin, M., Overton, J. M. C., Aspinall, R., et al. (2006). Making better biogeographical predictions of species' distributions. J. Appl. Ecol. 43, 386–392. doi: 10.1111/j.1365-2664.2006.01164.x
Guschina, I. A., and Harwood, J. L. (2006). Mechanisms of temperature adaptation in poikilotherms. FEBS Lett. 580, 5477–5483. doi: 10.1016/j.febslet.2006.06.066
Gutierrez-Marcos, J. F., and Dickinson, H. G. (2012). Epigenetic reprogramming in plant reproductive lineages. Plant Cell Physiol. 53, 817–823. doi: 10.1093/pcp/pcs052
Hanelt, D., Tüg, H., Bischof, K., GroÃÝ, C., Lippert, H., Sawall, T., et al. (2001). Light regime in an Arctic fjord: a study related to stratospheric ozone depletion as a basis for determination of UV efffects on algal growth. Mar. Biol. 138, 649–658. doi: 10.1007/s002270000481
Hansen, J., Sato, M., Ruedy, R., Lo, K., Lea, D. W., and Medina-Elizade, M. (2006). Global temperature change. Proc. Natl. Acad. Sci. U.S.A. 103, 14288–14293. doi: 10.1073/pnas.0606291103
Harley, C. D. G., Anderson, K. M., Demes, K. W., Jorve, J. P., Kordas, R. L., Coyle, T. A., et al. (2012). Effects of climate change on global seaweed communities. J. Phycol. 48, 1064–1078. doi: 10.1111/j.1529-8817.2012.01224.x
Hartog, C., and den Kuo, J. (2006). “Taxonomy and Biogeography of Seagrasses,” in Seagrasses: Biology, ecology and conservation, eds A. W. D. Larkum, R. J. Orth, and C. M. Duarte (Dordrecht: Springer), 1–23.
Hawkins, S. J., Moore, P. J., Burrows, M. T., Poloczanska, E., Mieszkowska, N., Herbert, R. J. H., et al. (2008). Complex interactions in a rapidly changing world: responses of rocky shore communities to recent climate change. Clim. Res. 37, 123–133. doi: 10.3354/cr00768
Hay, M. E., and Fenical, W. (1988). Marine plant-herbivore interactions: the ecology of chemical defense. Ann. Rev. Ecol. Syst. 19, 111–145. doi: 10.1146/annurev.es.19.110188.000551
Head, W. (1975). Nitrogen fixation with marine macroalga Codium fragile. Limnol. Oceanogr. 20, 815–823. doi: 10.4319/lo.1975.20.5.0815
Heck, K. L., and Valentine, J. F. (2006). Plant-herbivore interactions in seagrass meadows. J. Exp. Mar. Bio. Ecol. 330, 420–436. doi: 10.1016/j.jembe.2005.12.044
Herman, J. J., and Sultan, S. E. (2016). DNA methylation mediates genetic variation for adaptive transgenerational plasticity. Proc. R. Soc. B Biol. Sci. 283:20160988. doi: 10.1098/rspb.2016.0988
Hernán, G., Ramajo, L., Basso, L., Delgado, A., Terrados, J., Duarte, C. M., et al. (2016). Seagrass (Posidonia oceanica) seedlings in a high-CO2 world : from physiology to herbivory. Sci. Rep. 6:38017. doi: 10.1038/srep38017
Hernandez-Agreda, A., Gates, R. D., and Ainsworth, T. D. (2017). Defining the core microbiome in corals microbial soup. Trends Microbiol. 25, 125–140. doi: 10.1016/j.tim.2016.11.003
Herrera, C. M., and Bazaga, P. (2011). Untangling individual variation in natural populations: ecological, genetic and epigenetic correlates of long-term inequality in herbivory. Mol. Ecol. 20, 1675–1688. doi: 10.1111/j.1365-294X.2011.05026.x
Herrera, C. M., and Bazaga, P. (2016). Genetic and epigenetic divergence between disturbed and undisturbed subpopulations of a Mediterranean shrub: a 20-year field experiment. Ecol. Evol. 6, 3832–3847. doi: 10.1002/ece3.2161
Hirsch, S., Baumberger, R., and Grossniklaus, U. (2013). Epigenetic variation, inheritance, and selection in plant populations. Cold Spring Harb. Symp. Quant. Biol. 77, 97–104. doi: 10.1101/sqb.2013.77.014605
Hixson, S. M., and Arts, M. T. (2016). Climate warming is predicted to reduce omega-3, long-chain, polyunsaturated fatty acid production in phytoplankton. Glob. Chang. Biol. 22, 2744–2755. doi: 10.1111/gcb.13295
Hofmann, G. E. (2017). Ecological epigenetics in marine metazoans. Front. Mar. Sci. 4:4. doi: 10.3389/fmars.2017.00004
Holeski, L. M., Jander, G., and Agrawal, A. A. (2012). Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol. Evol. 27, 618–626. doi: 10.1016/j.tree.2012.07.011
Hollants, J., Leliaert, F., De Clerck, O., and Willems, A. (2013). What we can learn from sushi: a review on seaweed-bacterial associations. FEMS Microbiol. Ecol. 83, 1–16. doi: 10.1111/j.1574-6941.2012.01446.x
Holmer, M., and Bondgaard, E. J. (2001). Photosynthetic and growth response of eelgrass to low oxygen and high sulfide concentrations during hypoxic events. Aquat. Bot. 70, 29–38. doi: 10.1016/S0304-3770(00)00142-X
Holmer, M., and Hasler-Sheetal, H. (2014). Sulfide intrusion in seagrasses assessed by stable sulfur isotopes - a synthesis of current results. Front. Mar. Sci. 1:64. doi: 10.3389/fmars.2014.00064
Hooper, R. G. (1984). Functional adaptations to the polar environment by the Arctic kelp, Laminaria solidungula. Br. Phycol. J. 19:194.
Hootsmans, M. J. M., Vermaat, J. E., and Van Vierssen, W. (1987). Seed-bank development, germination and early seedling survival of two seagrass species from the Netherlands: Zostera marina L. and Zostera noltii hornem. Aquat. Bot. 28, 275–285.
Hughes, A. R., and Stachowicz, J. J. (2004). Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. Proc. Natl. Acad. Sci. U.S.A. 101, 8998–9002. doi: 10.1073/pnas.0402642101
Hyndes, G. A., Heck, K. L., Vergés, A., Harvey, E. S., Kendrick, G. A., Lavery, P. S., et al. (2016). Accelerating tropicalization and the transformation of temperate seagrass meadows. Bioscience 66, 938–945. doi: 10.1093/biosci/biw111
Illingworth, R. S., and Bird, A. P. (2009). CpG islands - “A rough guide.” FEBS Lett. 583, 1713–1720. doi: 10.1016/j.febslet.2009.04.012
Jackson, C., Salomaki, E. D., Lane, C. E., and Saunders, G. W. (2017). Kelp transcriptomes provide robust support for interfamilial relationships and revision of the little known Arthrothamnaceae (Laminariales). J. Phycol. 53, 1–6. doi: 10.1111/jpy.12465
Jenkins, S. R., Coleman, R. A., Della Santina, P., Hawkins, S. J., Burrows, M. T., and Hartnoll, R. G. (2005). Regional scale differences in the determinism of grazing effects in the rocky intertidal. Mar. Ecol. Prog. Ser. 287, 77–86. doi: 10.3354/meps287077
Ji, P., Zhang, Y., Wang, J., and Zhao, F. (2017). MetaSort untangles metagenome assembly by reducing microbial community complexity. Nat. Commun. 8:14306. doi: 10.1038/ncomms14306
Jinhua, P., Xin, J., Xiaojie, L. I., Yizhou, C., and Zhuangzhi, Z. (2011). Influence of temperature and salinity on germination of eelgrass (Zostera marina L.) Seeds. J. Ocean Univ. China 10:11802. doi: 10.1007/s11802-011-1800-y
Johansson, M. L., Alberto, F., Reed, D. C., Raimondi, P. T., Coelho, N. C., Young, M. A., et al. (2015). Seascape drivers of Macrocystis pyrifera population genetic structure in the northeast Pacific. Mol. Ecol. 24, 4866–4885. doi: 10.1111/mec.13371
Johansson, M. L., Raimondi, P. T., Reed, D. C., Coelho, N. C., Serrão, E. A., and Alberto, F. A. (2013). Looking into the black box: simulating the role of self-fertilization and mortality in the genetic structure of Macrocystis pyrifera. Mol. Ecol. 22, 4842–4854. doi: 10.1111/mec.12444
Joint, I., Tait, K., and Wheeler, G. (2007). Cross-kingdom signalling: exploitation of bacterial quorum sensing molecules by the green seaweed Ulva. Philos. Trans. R. Soc. B Biol. Sci. 362, 1223–1233. doi: 10.1098/rstb.2007.2047
Jueterbock, A., Boström, C., Reusch, T. B. H., Olsen, J. L., Kopp, M., Dhanasiri, A., et al. (2017). “Epigenetic variation in seagrass clones,” in 40 th New Phytologist Symposium, 10–11.
Jueterbock, A., Franssen, S. U., Bergmann, N., Gu, J., Coyer, J. A., Reusch, T. B. H., et al. (2016). Phylogeographic differentiation versus transcriptomic adaptation to warm temperatures in Zostera marina, a globally important seagrass. Mol. Ecol. 25, 5396–5411. doi: 10.1111/mec.13829
Jueterbock, A., Tyberghein, L., Verbruggen, H., Coyer, J. A., Olsen, J. L., and Hoarau, G. (2013). Climate change impact on seaweed meadow distribution in the North Atlantic rocky intertidal. Ecol. Evol. 3, 1356–1373. doi: 10.1002/ece3.541
Kaldy, J. E., Shafer, D. J., Ailstock, M. S., and Magoun, A. D. (2015). Effects of temperature, salinity and seed age on induction of Zostera japonica germination in. Aquat. Bot. 126, 73–79. doi: 10.1016/j.aquabot.2015.06.006
Kawashima, T., and Berger, F. (2014). Epigenetic reprogramming in plant sexual reproduction. Nat. Rev. Genet. 15, 613–624. doi: 10.1038/nrg3685
Kearney, M., Porter, W. P., Williams, C., Ritchie, S., and Hoffmann, A. A. (2009). Integrating biophysical models and evolutionary theory to predict climatic impacts on species' ranges: the dengue mosquito Aedes aegypti in Australia. Funct. Ecol. 23, 528–538. doi: 10.1111/j.1365-2435.2008.01538.x
Kelkar, N., Arthur, R., Marba, N., and Alcoverro, T. (2013). Green turtle herbivory dominates the fate of seagrass primary production in the Lakshadweep islands (Indian Ocean). Mar. Ecol. Prog. Ser. 485, 235–243. doi: 10.3354/meps10406
Keller, C. F. (2009). Global warming: a review of this mostly settled issue. Stoch. Environ. Res. Risk Assess. 23, 643–676. doi: 10.1007/s00477-008-0253-3
Keller, T. E., Lasky, J. R., and Yi, S. V. (2016). The multivariate association between genomewide DNA methylation and climate across the range of Arabidopsis thaliana. Mol. Ecol. 25, 1823–1837. doi: 10.1111/mec.13573
Kelly, N. M., Fonseca, M., and Whitfield, P. (2001). Predictive mapping for management and conservation of seagrass beds in North Carolina. Aquat. Conserv. Mar. Freshw. Ecosyst. 11, 437–451. doi: 10.1002/aqc.494
Kendrick, G. A., Waycott, M., Carruthers, T. J. B., Cambridge, M. L., Hovey, R., Krauss, S. L., et al. (2012). The Central Role of Dispersal in the Maintenance and Persistence of Seagrass Populations. Bioscience 62, 56–65. doi: 10.1525/bio.2012.62.1.10
Kilvitis, H. J., Hanson, H., Schrey, A. W., and Martin, L. B. (2017). Epigenetic potential as a mechanism of phenotypic plasticity in vertebrate range expansions. Integr. Comp. Biol. 57, 385–395. doi: 10.1093/icb/icx082
King, N. G., McKeown, N. J., Smale, D. A., and Moore, P. J. (in press). The importance of phenotypic plasticity local adaptation in driving intraspecific variability in thermal niches of marine macrophytes. Ecography doi: 10.1111/ecog.03186
Kivlin, S. N., Emery, S. M., and Rudgers, J. A. (2013). Fungal symbionts alter plant responses to global change. Am. J. Bot. 100, 1445–1457. doi: 10.3732/ajb.1200558
Klein Tank, A. M. G., Können, G. P., Tank, A. M. G. K., and Können, G. P. (2003). Trends in indices of daily temperature and precipitation extremes in Europe, 1946–99. J. Clim. 16, 3665–3680. doi: 10.1175/1520-0442(2003)016<3665:TIIODT>2.0.CO;2
Koch, M. S., and Erskine, J. M. (2001). Sulfide as a phytotoxin to the tropical seagrass Thalassia testudinum : interactions with light, salinity and temperature. J. Exper. Mar. Biol. Ecol. 266, 81–95. doi: 10.1016/S0022-0981(01)00339-2
Koch, M. S., Schopmeyer, S. A., Nielsen, O. I., Kyhn-Hansen, C., and Madden, C. J. (2007). Conceptual model of seagrass die-off in Florida Bay: links to biogeochemical processes. J. Exp. Mar. Bio. Ecol. 350, 73–88. doi: 10.1016/j.jembe.2007.05.031
Koch, M., Bowes, G., Ross, C., and Zhang, X. H. (2013). Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob. Chang. Biol. 19, 103–132. doi: 10.1111/j.1365-2486.2012.02791.x
Krause-Jensen, D., and Duarte, C. M. (2014). Expansion of vegetated coastal ecosystems in the future Arctic. Front. Mar. Sci. 1:77. doi: 10.3389/fmars.2014.00077
Krumhansl, K. A., Okamoto, D. K., Rassweiler, A., Novak, M., Bolton, J. J., Cavanaugh, K. C., et al. (2016). Global patterns of kelp forest change over the past half-century. Proc. Natl. Acad. Sci. U.S.A. 113, 13785–13790. doi: 10.1073/pnas.1606102113
Laird, P. W. (2010). Principles and challenges of genome-wide DNA methylation analysis. Nat. Rev. Genet. 11:191. doi: 10.1038/nrg2732
Lal, A., Arthur, R., Marbà, N., Lill, A. W. T., and Alcoverro, T. (2010). Implications of conserving an ecosystem modifier: increasing green turtle (Chelonia mydas) densities substantially alters seagrass meadows. Biol. Conserv. 143, 2730–2738. doi: 10.1016/j.biocon.2010.07.020
Lane, C. E., Mayes, C., Druehl, L. D., and Saunders, G. W. (2006). A multi-gene molecular investigation of the kelp (Laminariales, Phaeophyceae) supports substantial taxonomic re-organization. J. Phycol. 42, 493–512. doi: 10.1111/j.1529-8817.2006.00204.x
Larson, M. A., Thompson, F. R., Millspaugh, J. J., Dijak, W. D., and Shifley, S. R. (2004). Linking population viability, habitat suitability, and landscape simulation models for conservation planning. Ecol. Modell. 180, 103–118. doi: 10.1016/j.ecolmodel.2003.12.054
Latzel, V., Allan, E., Bortolini Silveira, A., Colot, V., Fischer, M., and Bossdorf, O. (2013). Epigenetic diversity increases the productivity and stability of plant populations. Nat. Commun. 4:2875. doi: 10.1038/ncomms3875
Latzel, V., Rendina González, A. P., and Rosenthal, J. (2016). Epigenetic memory as a basis for intelligent behavior in Clonal Plants. Front. Plant Sci. 7:1354. doi: 10.3389/fpls.2016.01354
Latzel, V., Zhang, Y., Karlsson Moritz, K., Fischer, M., and Bossdorf, O. (2012). Epigenetic variation in plant responses to defence hormones. Ann. Bot. 110, 1423–1428. doi: 10.1093/aob/mcs088
Laufkötter, C., Vogt, M., Gruber, N., Aita-Noguchi, M., Aumont, O., Bopp, L., et al. (2015). Drivers and uncertainties of future global marine primary production in marine ecosystem models. Biogeosciences 12, 6955–6984. doi: 10.5194/bg-12-6955-2015
Lavergne, S., Mouquet, N., Thuiller, W., and Ronce, O. (2010). Biodiversity and climate change: integrating evolutionary and ecological responses of species and communities. Annu. Rev. Ecol. Evol. Syst. 41, 321–350. doi: 10.1146/annurev-ecolsys-102209-144628
Leal, P. P., Hurd, C. L., Fernández, P. A., and Roleda, M. Y. (2017a). Meiospore development of the kelps Macrocystis pyrifera and Undaria pinnatifida under ocean acidification and ocean warming: independent effects are more important than their interaction. Mar. Biol. 164, 1–13. doi: 10.1007/s00227-016-3039-z
Leal, P. P., Hurd, C. L., Fernández, P. A., and Roleda, M. Y. (2017b). Ocean acidification and kelp development: reduced pH has no negative effects on meiospore germination and gametophyte development of Macrocystis pyrifera and Undaria pinnatifida. J. Phycol. 53, 557–566. doi: 10.1111/jpy.12518
Leipe, T., Dippner, J. W., Hille, S., Voss, M., Christiansen, C., and Bartholdy, J. (2008). Environmental changes in the central Baltic Sea during the past 1000 years: Inferences from sedimentary records, hydrography and climate. Oceanologia 50, 23–41.
Liew, Y. J., Zoccola, D., Li, Y., Tambutté, E., Venn, A. A., Michell, C. T., et al. (2017). Epigenome-associated phenotypic acclimatization to ocean acidification in a reef-building coral. bioRxiv 216:188227. doi: 10.1101/188227
Lirman, D., and Cropper, W. P. (2003). The influence of salinity on seagrass growth, survivorship, and distribution within Biscayne Bay, Florida: Field, experimental, and modeling studies. Estuaries 26, 131–141. doi: 10.1007/BF02691700
Liu, Q. A. (2013). The impact of climate change on plant epigenomes. Trends Genet. 29, 503–505. doi: 10.1016/j.tig.2013.06.004
Loques, F., Caye, G., and Meinesz, A. (1990). Germination in the marine phanerogam Zostera noltii Hornemann at Golfe Juan, French Mediterranean. Aquat. Bot. 38, 249–260.
Lorenzen, S. (2007). The limpet Patella vulgata L. at night in air: effective feeding on Ascophyllum nodosum monocultures and stranded seaweeds. J. Molluscan Stud. 73, 267–274. doi: 10.1093/mollus/eym022
Macedo, M. F., and Duarte, P. (2006). Phytoplankton production modelling in three marine ecosystems - Static versus dynamic approach. Ecol. Model. 190, 299–316. doi: 10.1016/j.ecolmodel.2005.05.012
Mancuso, F. P., D'Hondt, S., Willems, A., Airoldi, L., and De Clerck, O. (2016). Diversity and temporal dynamics of the epiphytic bacterial communities associated with the canopy-forming seaweed Cystoseira compressa (Esper) Gerloff and Nizamuddin. Front. Microbiol. 7:476 doi: 10.3389/fmicb.2016.00476
Marba, N., and Duarte, C. (1995). Coupling of Seagrass (Cymodocea Nodosa) patch dynamics to subaqueous dune migration author. J. Ecol. 83, 381–389.
Marquez, L. M., Redman, R. S., Rodriguez, R. J., and Roossinck, M. J. (2007). A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science 315, 513–515. doi: 10.1126/science.1136237
Marsh, A. G., and Pasqualone, A. A. (2014). DNA methylation and temperature stress in an Antarctic polychaete, Spiophanes tcherniai. Front. Physiol. 5:173. doi: 10.3389/fphys.2014.00173
Martin, B. C., Gleeson, D., Statton, J., Siebers, A. R., Grierson, P., Ryan, M. H., et al. (2018). Low light availability alters root exudation and reduces putative beneficial microorganisms in seagrass roots. Front. Microbiol. 8:2667. doi: 10.3389/fmicb.2017.02667
Martins, I., and Marques, J. C. (2002). A model for the growth of opportunistic Macroalgae (Enteromorpha sp.) in tidal estuaries. Estuar. Coast. Shelf Sci. 55, 247–257. doi: 10.1006/ecss.2001.0900
Martins, I., Lopes, R. J., Lillebø, A. I., Neto, J. M., Pardal, M. A., Ferreira, J. G., et al. (2007). Significant variations in the productivity of green macroalgae in a mesotidal estuary: implications to the nutrient loading of the system and the adjacent coastal area. Mar. Pollut. Bull. 54, 678–690. doi: 10.1016/j.marpolbul.2007.01.023
Martins, N., Tanttu, H., Pearson, G. A., Serrão, E. A., and Bartsch, I. (2017). Interactions of daylength, temperature and nutrients affect thresholds for life stage transitions in the kelp Laminaria digitata (Phaeophyceae). Bot. Mar. 60, 109–121. doi: 10.1515/bot-2016-0094
Matos, A. R., Hourton-Cabassa, C., Ciçek, D., Rezé, N., Arrabaça, J. D., Zachowski, A., et al. (2007). Alternative oxidase involvement in cold stress response of Arabidopsis thaliana fad2 and FAD3+ cell suspensions altered in membrane lipid composition. Plant Cell Physiol. 48, 856–865. doi: 10.1093/pcp/pcm061
Matsuo, Y., Suzuki, M., Kasai, H., Shizuri, Y., and Harayama, S. (2003). Isolation and phylogenetic characterization of bacteria capable of inducing differentiation in the green alga Monostroma oxyspermum. Environ. Microbiol. 5, 25–35. doi: 10.1046/j.1462-2920.2003.00382.x
Maumus, F., Rabinowicz, P., Bowler, C., and Rivarola, M. (2011). Stemming epigenetics in Marine Stramenopiles. Curr. Genomics 12, 357–370. doi: 10.2174/138920211796429727
McMinn, A., and Martin, A. (2013). Dark survival in a warming world. Proc. R. Soc. Biol. Sci. 280:20122909. doi: 10.1098/rspb.2012.2909
Michelou, V. K., Caporaso, J. G., Knight, R., and Palumbi, S. R. (2013). The ecology of microbial communities associated with Macrocystis pyrifera. PLoS ONE 8:e67480. doi: 10.1371/journal.pone.0067480
Minich, J., Morris, M., Brown, M., Doane, M., Edwards, M., Michael, T., et al. (2017). Elevated temperature drives kelp microbiome dysbiosis, while elevated carbon dioxide induces water microbiome disruption. PLoS ONE 13:192772. doi: 10.1371/journal.pone.0192772
Monastersky, R. (2013). Global carbon dioxide levels near worrisome milestone. Nature 497, 13–14. doi: 10.1038/497013a
Moore, K. A., Shields, E. C., Parrish, D. B., and Orth, R. J. (2012). Eelgrass survival in two contrasting systems: role of turbidity and summer water temperatures. Mar. Ecol. Prog. Ser. 448, 247–258. doi: 10.3354/meps09578
Moore, P., Hawkins, S. J., and Thompson, R. C. (2007). Role of biological habitat amelioration in altering the relative responses of congeneric species to climate change. Mar. Ecol. Prog. Ser. 334, 11–19. doi: 10.3354/meps334011
Mota, C. F., Engelen, A. H., Serrão, E. A., and Pearson, G. A. (2015). Some don't like it hot : microhabitat-dependent thermal and water stresses in a trailing edge population. Funct. Ecol. 29, 640–649. doi: 10.1111/1365-2435.12373
Müller, R., Laepple, T., Bartsch, I., and Wiencke, C. (2009). Impact of oceanic warming on the distribution of seaweeds in polar and cold-temperate waters. Bot. Mar. 52, 617–638. doi: 10.1515/BOT.2009.080
Murphy, H. T., and Lovett-Doust, J. (2007). Accounting for regional niche variation in habitat suitability models. Oikos 116, 99–110. doi: 10.1111/j.2006.0030-1299.15050.x
Neiva, J., Pearson, G. A., Valero, M., and Serrão, E. A. (2012). Fine-scale genetic breaks driven by historical range dynamics and ongoing density-barrier effects in the estuarine seaweed Fucus ceranoides L. BMC Evol. Biol. 12:78. doi: 10.1186/1471-2148-12-78
Neiva, J., Serrão, E. A., Assis, J., Pearson, G. A., Coyer, J. A., Olsen, J. L., et al. (2016). “Climate oscillations, range shifts and phylogeographic patterns of north atlantic fucaceae,” in Seaweed Phylogeography: Adaptation and Evolution of Seaweeds Under Environmental Change, eds Z. M. Hu and C. Fraser (Dordrecht: Springer), 279–308.
Nicastro, K. R., Zardi, G. I., Teixeira, S., Neiva, J., Serrão, E. A., and Pearson, G. A. (2013). Shift happens: trailing edge contraction associated with recent warming trends threatens a distinct genetic lineage in the marine macroalga Fucus vesiculosus. BMC Biol. 11:6. doi: 10.1186/1741-7007-11-6
Nicotra, A. B., Segal, D. L., Hoyle, G. L., Schrey, A. W., Verhoeven, K. J. F., and Richards, C. L. (2015). Adaptive plasticity and epigenetic variation in response to warming in an Alpine plant. Ecol. Evol. 5, 634–647. doi: 10.1002/ece3.1329
Olesen, B., Krause-Jensen, D., Marbà, N., and Christensen, P. B. (2015). Eelgrass Zostera marina in subarctic Greenland: dense meadows with slow biomass turnover in cold waters. Mar. Ecol. Prog. Ser. 518, 107–121. doi: 10.3354/meps11087
Olsen, J. L., Rouzé, P., Verhelst, B., Lin, Y.-C., Bayer, T., Collen, J., et al. (2016). The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature 530, 331–335. doi: 10.1038/nature16548
Olsen, Y. S., Potouroglou, M., Garcias-Bonet, N., and Duarte, C. M. (2014). Warming reduces pathogen pressure on a climate-vulnerable seagrass species. Estuar. Coasts 38, 659–667. doi: 10.1007/s12237-014-9847-9
Orth, R. J., Carruthers, T. J. B., Dennison, W. C., Duarte, C. M., Fourqurean, J. W., Heck, K. L., et al. (2006). A Global Crisis for Seagrass Ecosystems. Bioscience 56, 987–996. doi: 10.1641/0006-3568(2006)56[987:agcfse]2.0.co;2
Ortiz, M. (2008). Mass balanced and dynamic simulations of trophic models of kelp ecosystems near the Mejillones Peninsula of northern Chile (SE Pacific): comparative network structure and assessment of harvest strategies. Ecol. Model. 216, 31–46. doi: 10.1016/j.ecolmodel.2008.04.006
Pangesti, N., Pineda, A., Pieterse, C. M. J., Dicke, M., and van Loon, J. J. A. (2013). Two-way plant mediated interactions between root-associated microbes and insects: from ecology to mechanisms. Front. Plant Sci. 4:414. doi: 10.3389/fpls.2013.00414
Pearson, G. A., Lago-Leston, A., and Mota, C. (2009). Frayed at the edges: selective pressure and adaptive response to abiotic stressors are mismatched in low diversity edge populations. J. Ecol. 97, 450–462. doi: 10.1111/j.1365-2745.2009.01481.x
Pedersen, O., Binzer, T., and Borum, J. (2004). Sulphide intrusion in eelgrass (Zostera marina L.). Plant Cell Environ. 27, 595–602. doi: 10.1111/j.1365-3040.2004.01173.x
Penesyan, A., Marshall-Jones, Z., Holmstrom, C., Kjelleberg, S., and Egan, S. (2009). Antimicrobial activity observed among cultured marine epiphytic bacteria reflects their potential as a source of new drugs: research article. FEMS Microbiol. Ecol. 69, 113–124. doi: 10.1111/j.1574-6941.2009.00688.x
Pereira, T. R., Engelen, A. H., Pearson, G. A., Valero, M., and Serrão, E. A. (2015). Response of kelps from different latitudes to consecutive heat shock. J. Exper. Mar. Biol. Ecol. 463, 57–62. doi: 10.1016/j.jembe.2014.10.022
Phillips, R. C., Grant, W. S., and McRoy, C. P. (1983). Reproductive strategies of eelgrass (Zostera marina L.). Aquat. Bot. 16, 1–20.
Pieterse, C. M. J., Zamioudis, C., Berendsen, R. L., Weller, D. M., Van Wees, S. C. M., and Bakker, P. A. H. M. (2014). Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52, 347–375. doi: 10.1146/annurev-phyto-082712-102340
Plus, M., Chapelle, A., Ménesguen, A., Deslous-Paoli, J.-M., and Auby, I. (2003). Modelling seasonal dynamics of biomasses and nitrogen contents in a seagrass meadow (Zostera noltii Hornem.): application to the Thau lagoon (French Mediterranean coast). Ecol. Model. 161, 213–238. doi: 10.1016/S0304-3800(02)00350-2
Poloczanska, E. S., Brown, C. J., Sydeman, W. J., Kiessling, W., Schoeman, D. S., Moore, P. J., et al. (2013). Global imprint of climate change on marine life. Nat. Clim. Chang 3, 919–925. doi: 10.1038/nclimate1958
Procaccini, G., Olsen, J. L., and Reusch, T. B. H. (2007). Contribution of genetics and genomics to seagrass biology and conservation. J. Exp. Mar. Bio. Ecol. 350, 234–259. doi: 10.1016/j.jembe.2007.05.035
Prokopuk, L., Western, P. S., and Stringer, J. M. (2015). Transgenerational epigenetic inheritance: adaptation through the germline epigenome? Epigenomics 7, 829–846. doi: 10.2217/epi.15.36
Provasoli, L., and Pintner, I. J. (1980). Bacteria induced polymorphism in an axenic laboratory strain of Ulva Lactuca (Chlorophyceae). J. Phycol. 16, 196–201. doi: 10.1111/j.1529-8817.1980.tb03019.x
Pulido, C., and Borum, J. (2010). Eelgrass (Zostera marina) tolerance to anoxia. J. Exp. Mar. Bio. Ecol. 385, 8–13. doi: 10.1016/j.jembe.2010.01.014
Putnam, H. M., Davidson, J. M., and Gates, R. D. (2016). Ocean acidification influences host DNA methylation and phenotypic plasticity in environmentally susceptible corals. Evol. Appl. 9, 1165–1178. doi: 10.1111/eva.12408
Qu, J.-Q., Wang, X., Liu, C., LI, X.-L., and Zheng, X.-L. L. I. U. T (2013). Preliminary Analysis of DNA Methylationin of Saccharina japonica with MSAP. Period. Ocean Univ. China 10:8.
Quintero, I., and Wiens, J. J. (2013). Rates of projected climate change dramatically exceed past rates of climatic niche evolution among vertebrate species. Ecol. Lett. 16, 1095–1103. doi: 10.1111/ele.12144
Raimondi, A. P. T., Reed, D. C., Gaylord, B., and Washburn, L. (2011). Effects of Self-Fertilization in the Giant Kelp Macrocystis pyrifera. Ecology 85, 3267–3276. doi: 10.1890/03-0559
Rao, D., Webb, J. S., Holmström, C., Case, R., Low, A., Steinberg, P., et al. (2007). Low densities of epiphytic bacteria from the marine alga Ulva australis inhibit settlement of fouling organisms. Appl. Environ. Microbiol. 73, 7844–7852. doi: 10.1128/AEM.01543-07
Rasmann, S., De Vos, M., Casteel, C. L., Tian, D., Halitschke, R., Sun, J. Y., et al. (2012). Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol. 158, 854–863. doi: 10.1104/pp.111.187831
Reay, D., Sabine, C., Smith, P., and Hymus, G. (2007). Climate change 2007: spring-time for sinks. Nature 446, 727–728. doi: 10.1038/446727a
Reed, D. C., Raimondi, P. T., Washburn, L., Gaylord, B., Kinlan, B. P., and Drake, P. T. (2004a). “A metapopulation perspective on patch dynamics and connectivity in giant kelp,” in Marine Metapopulations, eds P. Sale and J. Kritzer (Millbrae, CA: Academic Press), 353–386.
Reed, D. C., Schroeter, S. C., and Raimondi, P. T. (2004b). Spore supply and habitat availability as sources of recruitment limitation in the giant kelp Macrocystis pyrifera (Phaeophyceae). J. Phycol. 40, 275–284. doi: 10.1046/j.1529-8817.2004.03119.x
Repolho, T., Duarte, B., Dionísio, G., Paula, J. R., Lopes, A. R., Rosa, I. C., et al. (2017). Seagrass ecophysiological performance under ocean warming and acidification. Sci. Rep. 7:41443. doi: 10.1038/srep41443
Reusch, T. B. H., and Boström, C. (2010). Widespread genetic mosaicism in the marine angiosperm Zostera marina is correlated with clonal reproduction. Evol. Ecol. 25, 899–913. doi: 10.1007/s10682-010-9436-8
Reusch, T. B. H., Boström, C., Stam, W. T., and Olsen, J. L. (1999). An ancient eelgrass clone in the Baltic. Mar. Ecol. Prog. Ser. 183, 301–304. doi: 10.3354/meps183301
Reusch, T. B. H., Ehlers, A., Hammerli, A., and Worm, B. (2005). Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc. Natl. Acad. Sci. U.S.A. 102, 2826–2831. doi: 10.1073/pnas.0500008102
Reusch, T. B. H., Stam, W. T., and Olsen, J. L. (2000). A microsatellite-based estimation of clonal diversity and population subdivision in Zostera marina, a marine flowering plant. Mol. Ecol. 9, 127–140. doi: 10.1046/j.1365-294X.2000.00839.x
Rey, O., Danchin, E., Mirouze, M., Loot, C., and Blanchet, S. (2016). Adaptation to global change: a transposable element-epigenetics perspective. Trends Ecol. Evol. 31, 514–526. doi: 10.1016/j.tree.2016.03.013
Richards, C. L., Alonso, C., Becker, C., Bossdorf, O., Bucher, E., Colome-Tatche, M., et al. (2017). Ecological plant epigenetics: evidence from model and non-model species, and the way forward. bioRxiv 5905. doi: 10.1101/130708
Roleda, M. Y. (2009). Photosynthetic response of Arctic kelp zoospores exposed to radiation and thermal stress. Photochem. Photobiol. Sci. 8:1302. doi: 10.1039/b901098j
Roleda, M. Y. (2016). Stress physiology and reproductive phenology of Arctic endemic kelp Laminaria solidungula J. Agardh. Polar Biol. 39, 1967–1977. doi: 10.1007/s00300-015-1813-x
Roleda, M. Y., Campana, G. L., Wiencke, C., Hanelt, D., Quartino, M. L., and Wulff, A. (2009). Sensitivity of antarctic Urospora penicilliformis (Ulotrichales, Chlorophyta) to ultraviolet radiation is life-stage dependent. J. Phycol. 45, 600–609. doi: 10.1111/j.1529-8817.2009.00691.x
Roleda, M. Y., Morris, J. N., McGraw, C. M., and Hurd, C. L. (2012). Ocean acidification and seaweed reproduction: increased CO2 ameliorates the negative effect of lowered pH on meiospore germination in the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae). Glob. Chang. Biol. 18, 854–864. doi: 10.1111/j.1365-2486.2011.02594.x
Roleda, M. Y., Van De Poll, W. H., Hanelt, D., and Wiencke, C. (2004). PAR and UVBR effects on photosynthesis, viability, growth and DNA in different life stages of two coexisting Gigartinales: implications for recruitment and zonation pattern. Mar. Ecol. Prog. Ser. 281, 37–50. doi: 10.3354/meps281037
Roleda, M. Y., Wiencke, C., Hanelt, D., and Bischof, K. (2007). Sensitivity of the early life stages of macroalgae from the Northern Hemisphere to ultraviolet radiation. Photochem. Photobiol. 83, 851–862. doi: 10.1562/2006-08-17-IR-1005
Romero, M., Martin-Cuadrado, A. B., Roca-Rivada, A., Cabello, A. M., and Otero, A. (2011). Quorum quenching in cultivable bacteria from dense marine coastal microbial communities. FEMS Microbiol. Ecol. 75, 205–217. doi: 10.1111/j.1574-6941.2010.01011.x
Rothman, M. D., Mattio, L., Anderson, R. J., and Bolton, J. J. (2017). A phylogeographic investigation of the kelp genus Laminaria (Laminariales, Phaeophyceae), with emphasis on the South Atlantic Ocean. J. Phycol. 53, 778–789. doi: 10.1111/jpy.12544
Rothman, M. D., Mattio, L., Wernberg, T., Anderson, R. J., Uwai, S., Mohring, M. B., et al. (2015). A molecular investigation of the genus Ecklonia (Phaeophyceae, Laminariales) with special focus on the Southern Hemisphere. J. Phycol. 51, 236–246. doi: 10.1111/jpy.12264
Ruiz, J. M., Marín-Guirao, L., García-Muñoz, R., Ramos-Segura, A., Bernardeau-Esteller, J., Pérez, M., et al. (in press). Experimental evidence of warming-induced flowering in the Mediterranean seagrass Posidonia oceanica. Mar. Pollut. Bull. doi: 10.1016/j.marpolbul.2017.10.037
Saada, G., Nicastro, K. R., Jacinto, R., Pearson, G. A., Mcquaid, C. D., Serr, E. A., et al. (2016). Taking the heat: distinct vulnerability to thermal stress of central and threatened peripheral lineages of a marine macroalga. J. Conserv. Biogeogr. 22, 1060–1068. doi: 10.1111/ddi.12474
Saderne, V., Fietzek, P., and Herman, P. M. J. (2013). Extreme variations of pCO2 and pH in a macrophyte meadow of the Baltic Sea in summer: evidence of the effect of photosynthesis and local upwelling. PLoS ONE 8:62689. doi: 10.1371/journal.pone.0062689
Saha, M., Rempt, M., Stratil, S. B., Wahl, M., Pohnert, G., and Weinberger, F. (2014). Defence chemistry modulation by light and temperature shifts and the resulting effects on associated epibacteria of Fucus vesiculosus. PLoS ONE 9:105333. doi: 10.1371/journal.pone.0105333
Saunders, M. I., Atkinson, S., Klein, C. J., Weber, T., and Possingham, H. P. (2017). Increased sediment loads cause non-linear decreases in seagrass suitable habitat extent. PLoS ONE 12:e0187284. doi: 10.1371/journal.pone.0187284
Schär, C., and Jendritzky, G. (2004). Hot news from summer 2003. News and views. Nature 432, 559–560. doi: 10.1038/432559a
Schiel, D. R., and Foster, M. S. (2006). The population biology of large brown seaweeds: ecological consequences of multiphase life histories in dynamic coastal environments. Annu. Rev. Ecol. Evol. Syst. 37, 343–372. doi: 10.1146/annurev.ecolsys.37.091305.110251
Schield, D. R., Walsh, M. R., Card, D. C., Andrew, A. L., Adams, R. H., and Castoe, T. A. (2016). EpiRADseq: scalable analysis of genomewide patterns of methylation using next-generation sequencing. Methods Ecol. Evol. 7, 60–69. doi: 10.1111/2041-210X.12435
Schlichting, C. D., and Wund, M. A. (2014). Phenotypic plasticity and epigenetic marking: an assessment of evidence for genetic accommodation. Evolution (NY). 68, 656–672. doi: 10.1111/evo.12348
Schmitz, R. J., He, Y., Valdés-López, O., Khan, S. M., Joshi, T., Urich, M. A., et al. (2013). Epigenome-wide inheritance of cytosine methylation variants in a recombinant inbred population. Genome Res. 23, 1663–1674. doi: 10.1101/gr.152538.112
Schrader, L., Kim, J. W., Ence, D., Zimin, A., Klein, A., Wyschetzki, K., et al. (2014). Transposable element islands facilitate adaptation to novel environments in an invasive species. Nat. Commun. 5:5495. doi: 10.1038/ncomms6495
Schrey, A. W., Alvarez, M., Foust, C. M., Kilvitis, H. J., Lee, J. D., Liebl, A. L., et al. (2013). Ecological epigenetics: beyond MS-AFLP. Integr. Comp. Biol. 53, 340–350. doi: 10.1093/icb/ict012
Schweinsberg, M., Weiss, L. C., Striewski, S., Tollrian, R., and Lampert, K. P. (2015). More than one genotype: how common is intracolonial genetic variability in scleractinian corals? Mol. Ecol. 24, 2673–2685. doi: 10.1111/mec.13200
Serra, I. A., Innocenti, A. M., Di Maida, G., Calvo, S., Migliaccio, M., Zambianchi, E., et al. (2010). Genetic structure in the Mediterranean seagrass Posidonia oceanica: disentangling past vicariance events from contemporary patterns of gene flow. Mol. Ecol. 19, 557–568. doi: 10.1111/j.1365-294X.2009.04462.x
Seymour, D. K., Koenig, D., Hagmann, J., Becker, C., and Weigel, D. (2014). Evolution of DNA Methylation Patterns in the Brassicaceae is Driven by Differences in Genome Organization. PLoS Genet. 10:e1004785. doi: 10.1371/journal.pgen.1004785
Short, F., Carruthers, T., Dennison, W., and Waycott, M. (2007). Global seagrass distribution and diversity: a bioregional model. J. Exp. Mar. Bio. Ecol. 350, 3–20. doi: 10.1016/j.jembe.2007.06.012
Silberhorn, G. M., Orth, R. J., and Moore, K. A. (1983). Anthesis and seed production in Zostera marina L. (eelgrass) from the chesepeak by. Aquat. Bot. 15, 133–144. doi: 10.1016/0304-3770(83)90024-4
Sinclair, S. J., White, M. D., and Newell, G. R. (2010). How useful are species distribution models for managing biodiversity under future climates? Ecol. Soc. 15:8.
Sinensky, M. (1974). Homeoviscous adaptation–a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 71, 522–525. doi: 10.1073/pnas.71.2.522
Singh, R. P., and Reddy, C. R. K. (2016). Unraveling the functions of the macroalgal microbiome. Front. Microbiol. 6:1488. doi: 10.3389/fmicb.2015.01488
Slotkin, R. K. (2016). Plant epigenetics: from genotype to phenotype and back again. Genome Biol. 17:57. doi: 10.1186/s13059-016-0920-5
Slotkin, R. K., and Martienssen, R. (2007). Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 8, 272–285. doi: 10.1038/nrg2072
Smale, D. A., Burrows, M. T., Moore, P., O'Connor, N., and Hawkins, S. J. (2013). Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol. Evol. 3, 4016–4038. doi: 10.1002/ece3.774
Solidoro, C., Pecenik, G., Pastres, R., Franco, D., and Dejak, C. (1997). Modelling macroalgae (Ulva rigida) in the venice lagoon: model structure identification and first parameters estimation. Ecol. Model. 94, 191–206. doi: 10.1016/S0304-3800(96)00025-7
Sorte, C. J. B., Fuller, A., and Bracken, M. E. S. (2010). Impacts of a simulated heat wave on composition of a marine community. Oikos 119, 1909–1918. doi: 10.1111/j.1600-0706.2010.18663.x
Spalding, M. D., Fox, H. E., Allen, G. R., Davidson, N., Ferdaña, Z. A., Finlayson, M., et al. (2007). Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57:573. doi: 10.1641/B570707
Srivastava, A., Guissé, B., Greppin, H., and Strasser, R. J. (1997). Regulation of antenna structure and electron transport in Photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim. Biophys. Acta Bioenerg. 1320, 95–106. doi: 10.1016/S0005-2728(97)00017-0
Staehr, P. A., and Wernberg, T. (2009). Physiological responses of Ecklonia radiata (Laminariales) to a latitudinal gradient in ocean temperature. J. Phycol. 45, 91–99. doi: 10.1111/j.1529-8817.2008.00635.x
Stapley, J., Santure, A. W., and Dennis, S. R. (2015). Transposable elements as agents of rapid adaptation may explain the genetic paradox of invasive species. Mol. Ecol. 24, 2241–2252. doi: 10.1111/mec.13089
Staton, S. E., and Burke, J. M. (2015). Evolutionary transitions in the Asteraceae coincide with marked shifts in transposable element abundance. BMC Genomics 16:623. doi: 10.1186/s12864-015-1830-8
Staufenberger, T., Thiel, V., Wiese, J., and Imhoff, J. F. (2008). Phylogenetic analysis of bacteria associated with Laminaria saccharina. FEMS Microbiol. Ecol. 64, 65–77. doi: 10.1111/j.1574-6941.2008.00445.x
Steneck, R. S., Graham, M. H., Bourque, B. J., Corbett, D., Erlandson, J. M., Estes, J. A., et al. (2002). Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ. Conserv. 29, 436–459. doi: 10.1017/S0376892902000322
Strasser, R. J., Srivastava, A., and Tsimilli-Michael, M. (2000). “The fluorescence transient as a tool to characterize and screen photosynthetic samples,” in Probing Photosynthesis: Mechanism, Regulation & Adaptation, 443–480. Available online at: http://www.hansatech-instruments.com/docs/the%20fluorescence%20transient.pdf/
Svendsen, H., Beszczynska-Møller, A., Hagen, J. O., Lefauconnier, B., Tverberg, V., Gerland, S., et al. (2002). The physical environment of Kongsfjorden – Krossfjorden, an Arctic fjord system in Svalbard. Polar Res. 21, 133–166. doi: 10.1111/j.1751-8369.2002.tb00072.x
Taylor, K. E., Stouffer, R. J., and Meehl, G. A. (2012). An overview of CMIP5 and the experiment design. Bull. Am. Meteorol. Soc. 93, 485–498. doi: 10.1175/BAMS-D-11-00094.1
Teagle, H., Hawkins, S. J., Moore, P. J., and Smale, D. A. (2017). The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Mar. Bio. Ecol. 492, 81–98. doi: 10.1016/j.jembe.2017.01.017
Tellier, F., Meynard, A. P., Correa, J. A., Faugeron, S., and Valero, M. (2009). Phylogeographic analyses of the 30°s south-east Pacific biogeographic transition zone establish the occurrence of a sharp genetic discontinuity in the kelp Lessonia nigrescens: vicariance or parapatry? Mol. Phylogenet. Evol. 53, 679–693. doi: 10.1016/j.ympev.2009.07.030
Tellier, F., Tapia, J., Faugeron, S., Destombe, C., and Valero, M. (2011). The Lessonia nigrescens species complex (Laminariales, Phaeophyceae) shows strict parapatry and complete reproductive isolation in a secondary contact zone. J. Phycol. 47, 894–903. doi: 10.1111/j.1529-8817.2011.01019.x
Thomson, J. A., Burkholder, D. A., Heithaus, M. R., Fourqurean, J. W., Fraser, M. W., Statton, J., et al. (2015). Extreme temperatures, foundation species, and abrupt ecosystem change : an example from an iconic seagrass ecosystem. Glob. Change Biol. 21, 1463–1474. doi: 10.1111/gcb.12694
Tirichine, L., and Bowler, C. (2011). Decoding algal genomes: tracing back the history of photosynthetic life on Earth. Plant J. 66, 45–57. doi: 10.1111/j.1365-313X.2011.04540.x
tom Dieck, I. (1989). Vergleichende Untersuchungen zur ökophysiologie und Kreuzbarkeit innerhalb der digitaten Sektion der Gattung Laminaria, Ph.D. thesis, University of Hamburg, Hamburg.
Triest, L., and Sierens, T. (2014). Seagrass radiation after Messinian salinity crisis reflected by strong genetic structuring and out-of-Africa scenario (Ruppiaceae). PLoS ONE 9:104264. doi: 10.1371/journal.pone.0104264
Trucchi, E., Mazzarella, A. B., Gilfillan, G. D., Lorenzo, M. T., Schönswetter, P., and Paun, O. (2016). BsRADseq: screening DNA methylation in natural populations of non-model species. Mol. Ecol. 25, 1697–1713. doi: 10.1111/mec.13550
Tyberghein, L., Verbruggen, H., Pauly, K., Troupin, C., Mineur, F., and De Clerck, O. (2012). Bio-ORACLE: a global environmental dataset for marine species distribution modelling. Glob. Ecol. Biogeogr. 21, 272–281. doi: 10.1111/j.1466-8238.2011.00656.x
Valle, M., Chust, G., del Campo, A., Wisz, M. S., Olsen, S. M., Garmendia, J. M., et al. (2014). Projecting future distribution of the seagrass Zostera noltii under global warming and sea level rise. Biol. Conserv. 170, 74–85. doi: 10.1016/j.biocon.2013.12.017
van der Heide, T., Govers, L. L., de Fouw, J., Olff, H., van der Geest, M., van Katwijk, M. M., et al. (2012). A three-stage symbiosis forms the foundation of seagrass ecosystems. Science 336, 1432–1434. doi: 10.1126/science.1219973
van Ginneken, V. J., Helsper, J. P., de Visser, W., van Keulen, H., and Brandenburg, W. A. (2011). Polyunsaturated fatty acids in various macroalgal species from north Atlantic and tropical seas. Lipids Health Dis. 10:104. doi: 10.1186/1476-511X-10-104
van Gurp, T. P., Wagemaker, N. C. A. M., Wouters, B., Vergeer, P., Ouborg, J. N. J., and Verhoeven, K. J. F. (2016). epiGBS: reference-free reduced representation bisulfite sequencing. Nat. Methods 13, 322–324. doi: 10.1038/nmeth.3763
van Vuuren, D. P., Edmonds, J., Kainuma, M., Riahi, K., Thomson, A., Hibbard, K., et al. (2011). The representative concentration pathways: an overview. Clim. Change 109, 5–31. doi: 10.1007/s10584-011-0148-z
Veluchamy, A., Lin, X., Maumus, F., Rivarola, M., Bhavsar, J., Creasy, T., et al. (2013). Insights into the role of DNA methylation in diatoms by genome-wide profiling in Phaeodactylum tricornutum. Nat. Commun. 4:2091. doi: 10.1038/ncomms3091
Vergés, A., Becerro, M. A., Alcoverro, T., and Romero, J. (2007). Experimental evidence of chemical deterrence against multiple herbivores in the seagrass Posidonia oceanica. Mar. Ecol. Prog. Ser. 343, 107–114. doi: 10.3354/meps06885
Vergés, A., Doropoulos, C., Malcolm, H. A., Skye, M., Garcia-Pizá, M., Marzinelli, E. M., et al. (2016). Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc. Natl. Acad. Sci. U.S.A. 113, 13791–13796. doi: 10.1073/pnas.1610725113
Vergés, A., Pérez, M., Alcoverro, T., and Romero, J. (2008). Compensation and resistance to herbivory in seagrasses: induced responses to simulated consumption by fish. Oecologia 155, 751–760. doi: 10.1007/s00442-007-0943-4
Vergés, A., Steinberg, P. D., Hay, M. E., Poore, A. G. B., Campbell, A. H., Ballesteros, E., et al. (2014). The tropicalization of temperate marine ecosystems : climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B Biol. Sci. 281, 1–10. doi: 10.1098/rspb.2014.0846
Verhoeven, K. J. F., and Preite, V. (2014). Epigenetic variation in asexually reproducing organisms. Evolution (NY). 68, 644–655. doi: 10.1111/evo.12320
Verhoeven, K. J. F., VonHoldt, B. M., and Sork, V. L. (2016). Epigenetics in ecology and evolution: what we know and what we need to know. Mol. Ecol. 25, 1631–1638. doi: 10.1111/mec.13617
Vieira, C., Engelen, A. H., Guentas, L., Aires, T., Houlbreque, F., Gaubert, J., et al. (2016). Species specificity of bacteria associated to the brown seaweeds Lobophora (Dictyotales, Phaeophyceae) and their potential for induction of rapid coral bleaching in Acropora muricata. Front. Microbiol. 7:316. doi: 10.3389/fmicb.2016.00316
Viejo, R. M., Martínez, B., Arrontes, J., Astudillo, C., and Hernández, L. (2011). Reproductive patterns in central and marginal populations of a large brown seaweed: drastic changes at the southern range limit. Ecography (Cop.). 34, 75–84. doi: 10.1111/j.1600-0587.2010.06365.x
Von elert, E. (2004). Food quality constraints in Daphnia : interspecific differences in the response to the absence of a long chain polyunsaturated fatty acid in the food source. Hydrobiologia 526, 187–196. doi: 10.1023/B:HYDR.0000041604.01529.00
Wada, K. C., and Takeno, K. (2010). Stress-induced flowering. Plant Signal. Behav. 5, 944–947. doi: 10.4161/psb.5.8.11826
Wahl, M., Jormalainen, V., Eriksson, B. K., Coyer, J. A., Molis, M., Schubert, H., et al. (2011). “Stress ecology in fucus: abiotic, biotic and genetic interactions,” in Advances in Marine Biology, ed M. Lesser (Academic Press), 37–105.
Wahl, M., Molis, M., Hobday, A. J., Dudgeon, S., Neumann, R., Steinberg, P., et al. (2015). The responses of brown macroalgae to environmental change from local to global scales: direct versus ecologically mediated effects. Perspect. Phycol. 2, 11–29. doi: 10.1127/pip/2015/0019
Wahl, M., Schneider Covachã, S., Saderne, V., Hiebenthal, C., Müller, J. D., Pansch, C., et al. (2017). Macroalgae may mitigate ocean acidification effects on mussel calcification by increasing pH and its fluctuations. Limnol. Oceanogr. 63, 3–21. doi: 10.1002/lno.10608
Wang, S., Lv, J., Zhang, L., Dou, J., Sun, Y., Li, X., et al. (2015). MethylRAD: a simple and scalable method for genome-wide DNA methylation profiling using methylation-dependent restriction enzymes. Open Biol. 5:150130. doi: 10.1098/rsob.150130
Waycott, M., Duarte, C. M., Carruthers, T. J. B., Orth, R. J., Dennison, W. C., Olyarnik, S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. U.S.A. 106, 12377–12381. doi: 10.1073/pnas.0905620106
Webster, N. S., and Reusch, T. B. H. (2017). Microbial contributions to the persistence of coral reefs. ISME J. 11, 2167–2174. doi: 10.1038/ismej.2017.66
Welsh, D. (2000). Nitrogen fixation in seagrass meadows : Regulation, plant ± bacteria interactions and significance to primary productivity. Ecol. Lett. 3, 58–71. doi: 10.1046/j.1461-0248.2000.00111.x
Wernberg, T., Russell, B. D., Thomsen, M. S., Gurgel, C. F. D., Bradshaw, C. J. A., Poloczanska, E. S., et al. (2011). Seaweed communities in retreat from ocean warming. Curr. Biol. 21, 1828–1832. doi: 10.1016/j.cub.2011.09.028
Wichard, T. (2015). Exploring bacteria-induced growth and morphogenesis in the green macroalga order Ulvales (Chlorophyta). Front. Plant Sci. 6:86. doi: 10.3389/fpls.2015.00086
Wiese, J., Thiel, V., Nagel, K., Staufenberger, T., and Imhoff, J. F. (2009). Diversity of antibiotic-active bacteria associated with the brown alga Laminaria saccharina from the baltic sea. Mar. Biotechnol. 11, 287–300. doi: 10.1007/s10126-008-9143-4
Winter, R., and Dzwolak, W. (2005). Exploring the temperature-pressure configurational landscape of biomolecules: from lipid membranes to proteins. Philos. Trans. A. Math. Phys. Eng. Sci. 363, 537–562. doi: 10.1098/rsta.2004.1507
Xie, M., and Yu, B. (2015). siRNA-directed DNA Methylation in Plants. Curr. Genomics 16, 23–31. doi: 10.2174/1389202915666141128002211
Yan, Z., Jones, P. D., Davies, T. D., Moberg, A., Bergström, H., Camuffo, D., et al. (2002). Trends of extreme temperatures in Europe and China based on daily observations. Clim. Change 53, 355–392. doi: 10.1023/A:1014939413284
York, P. H., Gruber, R. K., Hill, R., Ralph, P. J., Booth, D. J., and Macreadie, P. I. (2013). Physiological and morphological responses of the temperate seagrass Zostera muelleri to multiple stressors: investigating the interactive effects of light and temperature. PLoS ONE 8:76377. doi: 10.1371/journal.pone.0076377
Young, M., Ierodiaconou, D., and Womersley, T. (2015). Forests of the sea: predictive habitat modelling to assess the abundance of canopy forming kelp forests on temperate reefs. Remote Sens. Environ. 170, 178–187. doi: 10.1016/j.rse.2015.09.020
Zacher, K., Bernard, M., Bartsch, I., and Wiencke, C. (2016). Survival of early life history stages of Arctic kelps (Kongsfjorden, Svalbard) under multifactorial global change scenarios. Polar Biol. 39, 2009–2020. doi: 10.1007/s00300-016-1906-1
Zardi, G. I., Nicastro, K. R., Costa, J. F., Serrão, E. A., and Pearson, G. A. (2013). Estuarine, Coastal and Shelf Science Broad scale agreement between intertidal habitats and adaptive traits on a basis of contrasting population genetic structure. Estuar. Coast. Shelf Sci. 131, 140–148. doi: 10.1016/j.ecss.2013.08.016
Zhang, D., Zhang, Q. S., and Yang, X. Q. (2017). Adaptive strategies of Zostera japonica photosynthetic electron transport in response to thermal stress. Mar. Biol. 164, 1–12. doi: 10.1007/s00227-016-3064-y
Zhang, X., Yazaki, J., Sundaresan, A., Cokus, S., Chan, S. W. L., Chen, H., et al. (2006). Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell 126, 1189–1201. doi: 10.1016/j.cell.2006.08.003
Zhang, Y., and Jeltsch, A. (2010). The application of next generation sequencing in DNA methylation analysis. Genes (Basel). 1, 85–101. doi: 10.3390/genes1010085
Keywords: seagrasses, kelp forests, physiology, epigenetics, microbiome, modeling, early life stages, global climate change
Citation: Duarte B, Martins I, Rosa R, Matos AR, Roleda MY, Reusch TBH, Engelen AH, Serrão EA, Pearson GA, Marques JC, Caçador I, Duarte CM and Jueterbock A (2018) Climate Change Impacts on Seagrass Meadows and Macroalgal Forests: An Integrative Perspective on Acclimation and Adaptation Potential. Front. Mar. Sci. 5:190. doi: 10.3389/fmars.2018.00190
Received: 29 November 2017; Accepted: 11 May 2018;
Published: 04 June 2018.
Edited by:
Frédéric Gazeau, UMR7093 Laboratoire d'océanographie de Villefranche (LOV), FranceReviewed by:
Fernando Tuya, Universidad de Las Palmas de Gran Canaria, SpainMartin Zimmer, Leibniz Centre for Tropical Marine Research (LG), Germany
Copyright © 2018 Duarte, Martins, Rosa, Matos, Roleda, Reusch, Engelen, Serrão, Pearson, Marques, Caçador, Duarte and Jueterbock. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bernardo Duarte, baduarte@fc.ul.pt
 Bernardo Duarte
Bernardo Duarte Irene Martins
Irene Martins Rui Rosa3
Rui Rosa3  Ana R. Matos
Ana R. Matos Thorsten B. H. Reusch
Thorsten B. H. Reusch Aschwin H. Engelen
Aschwin H. Engelen Ester A. Serrão
Ester A. Serrão Gareth A. Pearson
Gareth A. Pearson João C. Marques
João C. Marques Isabel Caçador
Isabel Caçador Carlos M. Duarte
Carlos M. Duarte Alexander Jueterbock
Alexander Jueterbock