Abstract
Nutrient limitation of oceanic primary production exerts a fundamental control on marine food webs and the flux of carbon into the deep ocean1. The extensive boundaries of the oligotrophic sub-tropical gyres collectively define the most extreme transition in ocean productivity, but little is known about nutrient limitation in these zones1,2,3,4. Here we present the results of full-factorial nutrient amendment experiments conducted at the eastern boundary of the South Atlantic gyre. We find extensive regions in which the addition of nitrogen or iron individually resulted in no significant phytoplankton growth over 48 hours. However, the addition of both nitrogen and iron increased concentrations of chlorophyll a by up to approximately 40-fold, led to diatom proliferation, and reduced community diversity. Once nitrogen–iron co-limitation had been alleviated, the addition of cobalt or cobalt-containing vitamin B12 could further enhance chlorophyll a yields by up to threefold. Our results suggest that nitrogen–iron co-limitation is pervasive in the ocean, with other micronutrients also approaching co-deficiency. Such multi-nutrient limitations potentially increase phytoplankton community diversity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Moore, C. M. et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 6, 701–710 (2013)
Morel, F. M. M ., Milligan, A. J. & Saito, M. A. in The Oceans and Marine Geochemistry: Treatise on Geochemistry (ed. Elderfield, H. ) Ch. 5 (Pergamon, 2006)
Behrenfeld, M. J. et al. Controls on tropical Pacific Ocean productivity revealed through nutrient stress diagnostics. Nature 442, 1025–1028 (2006)
Saito, M. A. et al. Multiple nutrient stresses at intersecting Pacific Ocean biomes detected by protein biomarkers. Science 345, 1173–1177 (2014)
Saito, M. A., Rocap, G. & Moffett, J. W. Production of cobalt binding ligands in a Synechococcus feature at the Costa Rica upwelling dome. Limnol. Oceanogr. 50, 279–290 (2005)
Bertrand, E. M. et al. Vitamin B12 and iron colimitation of phytoplankton growth in the Ross Sea. Limnol. Oceanogr. 52, 1079–1093 (2007)
Arrigo, K. R. Marine microorganisms and global nutrient cycles. Nature 437, 349–355 (2005)
Saito, M. A., Goepfert, T. J. & Ritt, J. T. Some thoughts on the concept of colimitation: three definitions and the importance of bioavailability. Limnol. Oceanogr. 53, 276–290 (2008)
Harpole, W. S. et al. Nutrient co-limitation of primary producer communities. Ecol. Lett. 14, 852–862 (2011)
Sperfeld, E., Raubenheimer, D. & Wacker, A. Bridging factorial and gradient concepts of resource co-limitation: towards a general framework applied to consumers. Ecol. Lett. 19, 201–215 (2016)
Tilman, D. Resource Competition and Community Structure (Princeton Univ. Press, 1982)
Schade, J. D. et al. A conceptual framework for ecosystem stoichiometry: balancing resource supply and demand. Oikos 109, 40–51 (2005)
Danger, M., Daufresne, T., Lucas, F., Pissard, S. & Lacroix, G. Does Liebig’s law of the minimum scale up from species to communities? Oikos 117, 1741–1751 (2008)
Noble, A. E. et al. Basin-scale inputs of cobalt, iron, and manganese from the Benguela–Angola front to the South Atlantic Ocean. Limnol. Oceanogr. 57, 989–1010 (2012)
Moore, C. M. et al. Relative influence of nitrogen and phosphorus availability on phytoplankton physiology and productivity in the oligotrophic sub-tropical North Atlantic Ocean. Limnol. Oceanogr. 53, 291–305 (2008)
Behrenfeld, M. J. & Milligan, A. J. Photophysiological expressions of iron stress in phytoplankton. Annu. Rev. Mar. Sci. 5, 217–246 (2013)
Mann, E. L. & Chisholm, S. W. Iron limits the cell division rate of Prochlorococcus in the eastern equatorial Pacific. Limnol. Oceanogr. 45, 1067–1076 (2000)
Landry, M. R. et al. Biological response to iron fertilization in the eastern equatorial Pacific (IronEx II). I. Microplankton community abundances and biomass. Mar. Ecol. Prog. Ser. 201, 27–42 (2000)
Harpole, W. S. et al. Addition of multiple limiting resources reduces grassland diversity. Nature 537, 93–96 (2016)
Interlandi, S. J. & Kilham, S. S. Limiting resources and the regulation of diversity in phytoplankton communities. Ecology 82, 1270–1282 (2001)
Li, W. K. W. Macroecological patterns of phytoplankton in the northwestern North Atlantic Ocean. Nature 419, 154–157 (2002)
Edwards, K. F., Klausmeier, C. A. & Litchman, E. Evidence for a three-way trade-off between nitrogen and phosphorus competitive abilities and cell size in phytoplankton. Ecology 92, 2085–2095 (2011)
Boyd, P. W. et al. Mesoscale iron enrichment experiments 1993–2005: synthesis and future directions. Science 315, 612–617 (2007)
Saito, M. A., Sigman, D. M. & Morel, F. M. M. The bioinorganic chemistry of the ancient ocean: the co-evolution of cyanobacterial metal requirements and biogeochemical cycles at the Archean–Proterozoic boundary? Inorg. Chim. Acta 356, 308–318 (2003)
Martinez, S., Yang, X., Bennett, B. & Holz, R. C. A cobalt-containing eukaryotic nitrile hydratase. Biochim. Biophys. Acta. 1865, 107–112 (2017)
Croft, M. T., Lawrence, A. D., Raux-Deery, E., Warren, M. J. & Smith, A. G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438, 90–93 (2005)
Bertrand, E. M. et al. Methionine synthase interreplacement in diatom cultures and communities: implications for the persistence of B12 use by eukaryotic phytoplankton. Limnol. Oceanogr. 58, 1431–1450 (2013)
Ward, B. A., Dutkiewicz, S., Moore, C. M. & Follows, M. J. Iron, phosphorus, and nitrogen supply ratios define the biogeography of nitrogen fixation. Limnol. Oceanogr. 58, 2059–2075 (2013)
Tagliabue, A. et al. How well do global ocean biogeochemistry models simulate dissolved iron distributions? Glob. Biogeochem. Cycles 30, 149–174 (2016)
Göthlich, L. & Oschlies, A. Phytoplankton niche generation by interspecific stoichiometric variation. Glob. Biogeochem. Cycles 26, GB2010 (2012)
Acknowledgements
We thank the captain, crew, and principal scientist (M. Frank) of the RV Meteor M121 cruise; J. Pampín Baro, J. C. Yong and C. Schlosser for cruise support; and K. Nachtigall, T. Klüver, C. Utermann and P. Streu for technical laboratory assistance. This work was funded by a Marie Skłodowska-Curie Postdoctoral European Fellowship awarded to T.J.B. (OceanLiNES; project ID 658035). Additional financial aid to T.J.B. and E.P.A. from the European Commission is acknowledged (OCEAN-CERTAIN; project ID 603773). The cruise was funded by the Deutsche Forschungsgemeinschaft (DFG). A.T. was supported by funding from the European Research Council (project ID 724289). Funding to T.J.B. and C.M.M. for participation in the 2016 US GEOTRACES/OCB synthesis workshop is acknowledged.
Author information
Authors and Affiliations
Contributions
T.J.B. conceived, designed and carried out the study, analysed the data, and wrote the first draft of the manuscript. C.M.M. and T.J.B. worked on subsequent drafts and improved the data analysis. E.P.A. co-led the research cruise and oversaw the nutrient analyses. A.E. oversaw the flow cytometry analyses. I.R. and T.J.B. analysed the trace metal concentrations. E.M.B. contributed to interpretation of results. A.T. provided, and helped interpret, the PISCES2 model output. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks M. Behrenfeld, T. M. Conway and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Phytoplankton responses to nutrient amendment at near-coastal sites.
a–l, Chlorophyll a biomass, community, and Fv/Fm changes in experiments 1 (a–f) and 11 (g–l). Dots represent treatment replicates, bars indicate the mean, and lines represent the range. Statistically indistinguishable means are labelled with the same letter (ANOVA and Fisher PLSD P ≤ 0.05; n.s., not significant). Nitrogen was excluded from the factorial owing to high ambient nitrate concentrations (determined on-ship).
Extended Data Figure 2 Responses of the nanophytoplankton community in the bioassay experiments.
Grey data points represent cell counts in replicate treatment bottles; bar heights and lines indicate the mean and range, respectively (n = 3; units: ×1,000 cells per ml). Statistically indistinguishable means are labelled with the same letter (ANOVA and Fisher PLSD P ≤ 0.05, n = 3). Horizontal lines indicate initial cell counts. Red data points represent chlorophyll a fluorescence per cell and blue data points represent total nanophytoplankton chlorophyll a fluorescence; that is, cell counts × cellular chlorophyll fluorescence (both have arbitrary units with different scales, lines indicate the range).
Extended Data Figure 3 Responses of the picophytoplankton community in the bioassay experiments.
Grey data points represent cell counts in replicate treatment bottles; bar heights and lines indicate the mean and range, respectively (n = 3; units: ×1,000 cells per ml). Statistically indistinguishable means are labelled with the same letter (ANOVA and Fisher PLSD P ≤ 0.05, n = 3; n.s., not significant). Horizontal lines indicate initial cell counts. Red data points represent chlorophyll a fluorescence per cell and blue data points represent total picophytoplankton chlorophyll a fluorescence; that is, cell counts × cellular chlorophyll fluorescence (both have arbitrary units with different scales, lines indicate the range).
Extended Data Figure 4 Responses of Synechococcus in the bioassay experiments.
Grey data points represent cell counts in replicate treatment bottles; bar heights and lines indicate the mean and range, respectively (n = 3; units: ×1,000 cells per ml). Statistically indistinguishable means are labelled with the same letter (ANOVA and Fisher PLSD P ≤ 0.05, n = 3). Horizontal lines indicate initial cell counts. Red data points represent chlorophyll a fluorescence per cell and blue data points represent total Synechococcus chlorophyll a fluorescence; that is, cell counts × cellular chlorophyll fluorescence (both have arbitrary units with different scales, lines indicate the range).
Extended Data Figure 5 Responses of Prochlorococcus in the bioassay experiments.
Grey data points represent cell counts in replicate treatment bottles; bar heights and lines indicate the mean and range, respectively (n = 3; units: ×1,000 cells per ml). Statistically indistinguishable means are labelled with the same letter (ANOVA and Fisher PLSD P ≤ 0.05, n = 3). Horizontal lines indicate initial cell counts. Red data points represent chlorophyll a fluorescence per cell and blue data points represent total Prochlorococcus chlorophyll a fluorescence; that is, cell counts × cellular chlorophyll fluorescence (both have arbitrary units with different scales, lines indicate the range).
Extended Data Figure 6 Fv/Fm responses to nutrient treatment.
Data points represent measurements from replicate treatment bottles; bar heights and lines indicate the mean and range, respectively. Statistically indistinguishable means are labelled with the same letter (ANOVA and Tukey HSD P ≤ 0.05, n = 3; n.s., not significant). Horizontal lines indicate initial conditions. Changes in the Fv/Fm ratio between initial (t = 0 h) and control (t = 48 h) time points probably reflect differential relaxation of photosystem II down regulation or photosystem II repair.
Extended Data Figure 7 Diel cycles in Fv/Fm measurements in offshore waters.
a–r, Diel cycles. Grey dots denote individual Fv/Fm (Fv′/Fm′ during daytime) measurements; blue line indicates the 100-point moving average. Data were blank-corrected using a mean blank value for all offshore surface waters. Light blue boundaries indicate the range generated when the blank is increased or reduced by the standard deviation of the measured blank values. Red line indicates photosynthetically available radiation. s, Map showing the data collection locations for a–r relative to the bioassay experiments.
Extended Data Figure 8 CHEMTAX-derived community assemblages (fractional contribution to total chlorophyll a).
a–h, Initial waters from experiments 1–7 and 11. i, j, Initial waters and selected treatments from experiments 9 (i) and 10 (j).
Extended Data Figure 9 Exponential Shannon–Wiener diversity indices for the experiments.
Indices calculated using flow cytometry cell counts (grey dots represent treatment replicates, bars represent the mean, and lines represent the range) or pigment-derived community (black dots; n = 1 and where available). Statistically indistinguishable means for flow cytometry-derived ESWI are labelled with the same letter (ANOVA and Fisher PLSD P ≤ 0.05, n = 3). Horizontal lines indicate initial conditions.
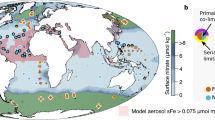
Extended Data Figure 10 Potential large-scale distribution of oceanic N–Fe co-limitation.
a, Global surface ocean as predicted using simulated nutrient fields from an ocean biogeochemical model run (PISCES2)29; co-limited regions (yellow grid cells) are assigned to grid cells with an N:Fe ratio falling in the range of N–Fe co-limited experiments (see Fig. 4a, b); N–Fe or Fe–N serially limited regions (that is, those approaching N–Fe co-limitation, grey grid cells) are assigned to grid cells with a N:Fe ratio falling in the range of N–Fe or Fe–N serially limited experiments. Large black dots show the locations where additional evidence of secondary or co-limitation between N and Fe has been found (see Supplementary Table 2 for details). Crosses are locations where nutrient enrichment experiments have been performed and where evidence was found for N (blue crosses) or Fe (red crosses) limitation (from synthesis by ref. 1). b, Observational N:Fe data gridded at the same resolution as the model. Observational Fe data29 have been combined with interpolated World Ocean Atlas nitrate for the location and month of the dissolved Fe measurement. c, Vertical domain of N:Fe ratios for a section of measured nutrient concentrations through the South Atlantic in austral summer (extended version of Fig. 1b; CoFeMUG cruise14). In the central gyre, the N supply from deeper waters is restricted by surface stratification, whereas subsurface waters are Fe-deficient relative to N, resulting from N remineralization and Fe scavenging. Large black dots indicate data points for which the measured N:Fe ratio was in the range in which we found N–Fe co-limitation; grey dots are within bounds of measured secondary N–Fe or Fe–N limitation. For a and c, thresholds at which N and Fe concentrations are both characterized as replete, regardless of the N:Fe ratio, have been applied; these are the maximum N or Fe concentrations in Supplementary Table 1 at which serial or co-limitation was found.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-4, a Supplementary Discussion, Supplementary Methods and Supplementary References. (PDF 888 kb)
Rights and permissions
About this article
Cite this article
Browning, T., Achterberg, E., Rapp, I. et al. Nutrient co-limitation at the boundary of an oceanic gyre. Nature 551, 242–246 (2017). https://doi.org/10.1038/nature24063
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature24063
This article is cited by
-
Roles and sources of B vitamins in the marine ecosystem
Reviews in Fish Biology and Fisheries (2024)
-
Temporal variation in vitamin B12 concentration and their impact on phytoplankton composition of surface waters of a coastal ocean off Japan (Ariake Sea)
Journal of Oceanography (2024)
-
Plastid-localized xanthorhodopsin increases diatom biomass and ecosystem productivity in iron-limited surface oceans
Nature Microbiology (2023)
-
Persistent equatorial Pacific iron limitation under ENSO forcing
Nature (2023)
-
Global analysis of ocean phytoplankton nutrient limitation reveals high prevalence of co-limitation
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.