Abstract
We studied the potential of a recently introduced species, the Asian brush-clawed crab (Hemigrapsus takanoi), to expand its distribution range further into the Baltic Sea. H. takanoi has been documented in the southwestern Baltic Sea since 2014. The ability to persist and further expand into the Baltic Proper will depend on their potential to sustain all stages of their complex life cycle, including pelagic larvae, under the Baltic Sea's conditions. Range limits may be established by the tolerance to low salinity, which in addition may be affected by water temperature. A key question is whether local populations at the distribution limit (within the Baltic Sea) show increased tolerance to low salinities and hence promote further expansion. We quantified the combined effects of salinity (10–33 PSU) and temperature (15–24 °C) on larval development in four populations of H. takanoi (two from the Baltic and two from the North Sea). We found substantial differences in larval performance between the populations from the Baltic and North Seas. Larvae from the North Sea populations always showed higher survival and faster development compared with those from the Baltic Sea. Only weak evidence of elevated tolerance towards low salinity was found in the larvae from the Baltic Sea populations. In addition, larvae from the population located near the range limit showed very low survival under all tested salinity-temperature combinations and no evidence of increased tolerance to low salinity. There was no apparent genetic differentiation among the studied populations in the mitochondrial cytochrome c oxidase subunit one gene (COI) implying high connectivity among the populations. In conclusion, the weak evidence of low salinity tolerance in Baltic Sea populations, and poor larval performance for the population located near the range limit, coupled with limited genetic differentiation suggest that subsidies are needed for populations to persist near the range limit. Alternatively, ontogenetic migrations would be required to sustain those populations. Monitoring efforts are needed to elucidate the underlaying mechanisms and document potential future range expansions.
Similar content being viewed by others
Introduction
Human-mediated climate change (Poloczanska et al. 2013; García Molinos et al. 2016; Boersma et al. 2016; Burrows et al. 2019) and widespread biological invasions (Gurevitch et al. 2011; Chan and Briski 2017) have major impacts on marine ecosystems. Invasive alien species (IAS) are an important component of global environmental change. IAS may cause modifications in community composition, and can be a major driver of local biodiversity loss (Bellard et al. 2016) as well as economic loss (Haubrock et al. 2021a, b; Henry et al. 2023). Climate change is considered an important driver of the success of biological invaders in aquatic ecosystems (Sorte et al. 2013; Gallardo et al. 2016); hence understanding the mechanisms of range expansion has become a priority (Gurevitch et al. 2011). The successful conquest of new habitats should depend fundamentally on ecological and evolutionary processes (Gurevitch et al. 2011) driven by the physical characteristics of the new habitat as well as the traits and performance exhibited by established species which are potential competitors, predators or parasites.
Long term data series (e.g. Boersma et al. 2016; de Amorim and Wiltshire et al. 2023), show that global change in marine ecosystems affects environmental drivers such as water temperature, salinity, pH, and oxygen content (Poloczanska et al. 2013; García Molinos et al. 2016; Boyd et al. 2018). However, future fluctuations in environmental factors as a consequence of human influences will differ between open ocean, estuarine, and near-shore sites with coastal areas typically experiencing larger fluctuations (Duarte 2007; Hofmann et al. 2011). Semi-enclosed seas such as the Baltic and North Seas are particularly susceptible to the effects of global change and are expected to be increasingly affected by rising surface temperatures and freshening by increased river runoff (Gräwe et al. 2013; Hiddink et al. 2015; Robins et al. 2016). The Baltic Sea, for example, was recently dubbed a “time machine” for the future coastal ocean (Reusch et al. 2018). It is characterised by relatively low salinity, which varies spatially and temporally, depending on the proximity to the North Sea, river inflow, freshwater runoff, precipitation, and seawater inflow events through Skagerrak and Kattegat (Lehmann et al. 2022). Typically, in the Baltic Sea, the salinity decreases from west to east (Lehmann et al. 2022). Therefore, considering the expected changes of salinity and temperature increase, organisms living in coastal habitats and regions of freshwater influence, will face new combinations of environmental drivers. Furthermore, intertidal ecosystems, e. g. in the North Sea, have already shown particularly pronounced and rapid changes in response to anthropogenic influences so that focusing research efforts on the study of intertidal organisms in general can provide new insights into the physiological effects of global ocean change (Somero 2002; Helmuth et al. 2006a, b).
An example of a recent introduction and expansion in coastal-estuarine areas is given by the Asian brush-clawed shore crab (Hemigrapsus takanoi), originated from southwestern Asia. H. takanoi has been reported in the coast of Northern Europe and is currently distributed from the English Channel to the SW Baltic Sea. This species is currently considered an ecological threat in European waters, exhibiting a potentially population-destabilising functional response towards blue mussels under Baltic Sea conditions (Theurich et al. 2022) but more studies are needed to confirm if H. takanoi is invasive in the European waters. H. takanoi was first found in Europe in 1993 on a ship’s hull in Bremerhaven, Germany (Gollasch 1999) and the first individuals were recorded in 1994, in the intertidal in La Rochelle, France (Noël et al. 1997). In the Wadden Sea, H. takanoi was first discovered in the Netherlands and later in Germany and Denmark (Geburzi et al. 2015). In the Baltic Sea, it was recorded from 2014 onwards, first in the south-west (Kiel fjord) and then further east (Mecklenburg Bight) and north (Skagerrak and Kattegat) (Geburzi et al. 2015, 2020; GBIF 2022).
As in many marine invertebrates, larval stages of H. takanoi are likely to play a central role in the process of invasion, as a contributor of the propagule pressure (Johnston et al. 2009). This is highlighted by the fact that larvae of H. takanoi have been found in ballast water of ships in the Arctic archipelago of Svalbard (Ware et al. 2016). Populations of sea bottom (= benthic) marine invertebrates, such as H. takanoi, are considered “open” in the sense that they are structured as a series of local populations of adult stages connected through larval dispersal (Caley et al. 1996; Armsworth 2002). Larvae play a central role in population connectivity and recovery from disturbance (Cowen et al. 2006; Giménez et al. 2020); yet, larval stages are more sensitive to variations in environmental conditions than adults are. Hence, in scenarios of environmental change, larvae may represent a bottleneck for population persistence (Sorte et al. 2010, 2018; Pandori and Sorte 2019). Theory predicts that at distribution limits, narrow ranges of larval tolerance may result in populations not being self-sustaining and instead being subsidised by human-mediated dispersal or natural dispersal on years when conditions are appropriate (Dauphinais et al. 2018; Giménez et al. 2020). A central point for the establishment of H. takanoi concerns the capacity of the early stages to tolerate thermal and salinity conditions and hence develop self-sustaining populations across the North-Baltic Sea gradient. We currently have information on the larval tolerance to temperature and salinity for two populations of H. takanoi (Japan: Mingkid et al. 2006; Kiel fjord, SW Baltic Sea: Nour et al. 2021, 2022). However, recent studies have highlighted important variability in environmental tolerance among invertebrate larvae from different females (Durrant et al. 2013; Applebaum et al. 2014; Spitzner et al. 2019) and among populations distributed over environmental gradients (Sanford et al. 2006; Nasrolahi et al. 2012; Baldanzi et al. 2018; Šargač et al. 2021). In particular, for H. takanoi, the current data on the lower limit of salinity tolerance (15 PSU) does not match the observed distribution of local adult populations: large numbers of adult crabs are found in areas of the Baltic Sea characterised by salinities below the known larval tolerance limit (Fig. 1a), e.g. Kiel fjord (salinity ~ 15 PSU) or Neustadt (salinity ~ 10 PSU) (Geburzi et al. 2020; Nour et al. 2020). Prior research only examined salinity tolerance at a single temperature (24 °C, Mingkid et al. 2006, 20 °C, Nour et al. 2021) or at two temperatures (19 and 23 °C, Nour et al. 2022) but ignored the interactive effects of temperature and salinity. Multiple-driver studies in the native crab Carcinus maenas showed interactive effects of salinity and temperature where negative effects of low salinity on survival are mitigated at high temperatures (abbreviated as TMLS) (Spitzner et al. 2019; Šargač et al. 2021; Torres et al. 2021a). To account for interactive effects and future warming scenarios, in this work, we used a mechanistic approach (sensu Boyd et al. 2018) to assess the response to low salinity in a multiple-driver set-up. In addition, there seems to be a high level of genetic diversity within populations of H. takanoi from both the North and Baltic Seas (Geburzi et al. 2020) that could be reflected in variability in responses to temperature and salinity among larvae from different females. Henceforth, it is essential to quantify larval performance in larvae from different females and populations located both at and away from the edge of the distribution range, as well as quantifying the genetic differentiation among those specific populations.
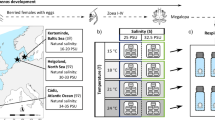
a Map of the collection sites (yellow triangles) for the four tested populations on Helgoland and Sylt (North Sea), and Kiel and Neustadt (Baltic Sea); blue points represent presence data from GBIF.org (GBIF 2022). b Experimental design to study the responses of larvae of H. takanoi from four different populations to different salinity and temperature conditions. Larvae of H.takanoi were reared from hatching to megalopa at four different temperatures: 15, 18, 21, and 24 °C, represented in the picture from light red (15 °C) to darker red (24 °C) and 5 salinities: 10, 15, 20, 25, 33 PSU represented by the borders from grey (10 PSU) to dark blue (33 PSU). Larvae were reared in 5 replicates of 10 individuals each
Therefore, we carried out a multi-population study of H. takanoi, by focusing on populations of the North Sea and SW Baltic Sea. To assess potential local adaptations to low salinity we included a population located near the distribution limit in the Baltic Sea (Neustadt) and the first established Baltic Sea population (Kiel) (Fig. 1a). We compared those to two North Sea island populations (Helgoland and Sylt) that are exposed to seawater conditions year-round. We quantified the larval responses to temperature and salinity and determined the genetic structure of the populations under study. The experiments on larval responses aimed at: (1) Determining the combination of temperature and salinity regimes that allows for larval survival and development. (2) Quantifying interactive effects of the temperature and salinity, at thermal ranges that are also expected from future climate change. The quantification of genetic variability may be an indicator of the degree of differentiation between the populations under study. Specifically, genetic homogeneity in concert with differences in larval performance would suggest either maternal effects or rapid local adaptation across the introduced range.
Materials and methods
Female collection and maintenance, larval rearing
Ovigerous females of Hemigrapsus takanoi (average carapace widths: Helgoland 14.4 ± 0.4 mm, Sylt 14.8 ± 1.7 mm, Kiel 14.6 ± 1.0 mm, Neustadt 18.0 ± 1.5 mm) were collected from four locations of the North and Baltic Seas coastal areas during summer (July 2021) in the middle of one reproductive period (Fig. 1a). During low tide, ovigerous females from the North Sea were collected by hand in the intertidal, from under rocks, small boulders, and mussel beds at the islands of Helgoland and Sylt (seawater conditions: 33 PSU). Ovigerous females from the Baltic Sea (Kiel and Neustadt) were collected by scraping the fouling communities in and close to marinas using a scraping scoop (mesh size 0.5 mm). Females were found in water depths of 1–2 m in clumps of blue mussel (Mytilus edulis) on the floor on fine sediment or on artificial walls e.g. of floating pontoons and waterside promenades in Baltic Sea water (Kiel: 15 PSU and Neustadt: 9.5 PSU). After collection, females (from Sylt, Kiel, and Neustadt) were transported to the laboratory on Helgoland (Alfred-Wegener-Institut, Helmholtz-Zentrum für Polar- und Meeresforschung, Biologische Anstalt Helgoland) in individual 1 L-containers filled with 500 ml water from the collection site. The individual containers were placed inside Coleman® coolers to ensure temperature stability during transport. Earlier studies showed that transport stress is negligible with this procedure (Šargač et al. 2021, 2022), thus no transport stress was simulated for animals collected on Helgoland.
In the laboratory, females were kept until hatching at 18 °C in individual 5 L-aquaria in natural UV-treated water at salinities corresponding to those at their respective sampling site: Helgoland & Sylt (33 PSU), Kiel (15 PSU) and Neustadt (10 PSU) and a 12:12 h light–dark cycle. Females were fed with shrimps (Crangon crangon) twice per week; water was changed daily to ensure high water quality at hatching. To minimise the effects of the field embryonic environment, we only used larvae that hatched later than 5 days after collection. In addition, to minimise the effect of the laboratory embryonic environment (i.e. maintenance time of females in the laboratory) we only used larvae that hatched within 35 days of collection (most of the females released larvae after 10–20 days). Variations in incubation time would not affect our results with regards to the combined effects of temperature and salinity because of the orthogonal nature of the experiment. However, such variation could drive population effects. Hence, for every combination of temperature and salinity, we checked through Pearson correlations if incubation time explained variations in performance of larvae from different females and populations (i.e. survival, duration of development, and growth rates: Figs. S1–S4). Most correlations were not significant and explained a very low percentage of variation in larval performance (exceptions: three cases, Figs. S1 and S4). Instead, we found important variations in larval performance for females releasing larvae after similar incubation times as well as small variations among larvae released after different incubation times (e.g. Fig. S1). Hence, we did not consider incubation time as a response variable any further. In addition, as a part of the design, we maintained all females at the salinity and temperature conditions measured at the time of collection at the habitat of origin (Fig. 1). Both maintenance of the females and larval rearing were performed following standard rearing techniques (Torres et al. 2021b).
Freshly hatched larvae from each female were assigned randomly to one of 20 treatments. The experimental design consisted of a factorial design based on the combination of 4 temperatures: 15, 18, 21, and 24 °C and 5 salinities: 10, 15, 20, 25, and 33 PSU (Fig. 1b). The chosen salinities range from the salinity found in Neustadt (10 PSU) to that found in Helgoland and Sylt (33 PSU). The chosen temperatures cover the range of temperatures typical for summer and those expected due to warming (Belkin 2009; Gräwe et al. 2013; Reusch et al. 2018; de Amorim and Wiltshire et al. 2023).
Survival and duration of development, dry mass, carbon and nitrogen content, and growth rates were assessed after exposure to the experimental conditions mentioned above. Each treatment consisted of five replicates (60 ml glass-beakers): ten larvae were randomly allocated to each replicate beaker. Experiments were repeated with larvae originated from 13 different females (i.e., Sylt: 4, Helgoland: 3, Kiel: 3, and Neustadt: 3). The position of the experimental vials was changed within each temperature-controlled room after each daily water change.
Larval rearing was performed in four temperature-controlled rooms (± 1 °C); the rooms are part of an array of temperature-controlled rooms of the same size at the Marine Station of Helgoland, constructed with identical materials, temperature regulation systems, similar type of shelves and lighting conditions (set to a 12:12 h light–dark cycle) that ensure identical rearing conditions to avoid confounding effects. Natural seawater was UV-treated, filtered (2 µm mesh size), and aerated seawater was used for the experiments. Experimental salinities were obtained by diluting seawater with appropriate amounts of tap water. Water and food (freshly hatched Artemia sp. nauplii ad libitum, Great Salt Lake Artemia) were changed daily. During the daily water change, dead larvae and moults were discarded and the larval stage of survivors was determined.
Elemental analysis
We quantified dry mass and elemental composition (i.e., carbon and nitrogen content) for freshly hatched zoea I and freshly moulted megalopa. For each experiment (i.e., for larvae from each female of origin), we sampled 3 replicates of 50 zoea I, as well as all the obtained megalopa. Furthermore, freshly hatched zoea I originated from additional females were also sampled (giving the following total number of females: Helgoland: 6; Sylt: 6; Kiel: 6; Neustadt: 4). During sampling, larvae were rinsed with distilled water, gently blotted dry with tissue (Kimtech® delicate task wipes), placed in a pre-weighted tin cartridge and stored at − 20 °C for further analysis. To quantify the dry mass, samples were freeze-dried for 48 h (Christ Alpha 1–4 freeze-drier) and weighed using a microbalance (Cubis ® MCA2.75S-2SOO-M Sartorius Lab Instruments GmbH & Co. KG). Carbon and nitrogen content were determined using an elemental analyzer (Vario MICRO cube CHNS analyser, Elementar Analysensysteme). In decapod crustaceans, carbon content is considered a proxy for lipids reserves, while nitrogen is the proxy for protein content (Dawirs 1986; Dawirs et al. 1986; Anger and Harms 1990).
Population genetics analyses (DNA extraction, amplification, and sequencing)
We amplified and sequenced a 618-base-pair (bp) fragment of the mitochondrial cytochrome oxidase subunit I (COI) gene, using the universal primers (LCO1490 and HC02198, Folmer et al. 1994). Sequences were obtained from 68 crabs (Helgoland: 6, Sylt: 24, Kiel: 19, Neustadt: 19) that were preserved in 97% ethanol and stored at − 20 °C prior to molecular analysis. Total DNA was extracted from pereiopod muscle tissue using the Qiagen DNeasy® Blood & Tissue kit following the manufacturer’s protocol for tissue samples except for the last step where DNA was eluded by adding 100 µl of elution buffer and centrifuged at 10,000 rpm. DNA purity and concentration were assessed using the Nanodrop Spectrophotometer (NanoDrop ONE ThermoFisher). DNA was used as a template for Polymerase chain reaction (PCR) amplification using PuReTaq® Ready-To-Go™ polymerase (Cytiva). All PCRs were carried out in a 25 µl reaction mix containing 2 µl DNA, 1 µl BSA and, 2.5 µl (5 µM) of each forward and reverse primer. The amplifications were carried out by a (Mastercylcler®) Eppendorf thermocycler with a program that consisted of 2 min at 94 °C followed by 35 cycles of 0.5 min at 94 °C, 1 min at 50 °C and 1 min at 72 °C, and a final extension of 10 min at 72 °C. The PCR products were Sanger sequenced in forward and reverse direction at the Institute of Clinical Molecular Biology in Kiel (IKMB).
Data analysis
The response variables were cumulative survival, duration of development, instantaneous growth rate, and body mass and elemental composition at metamorphosis. Cumulative survival was calculated as the proportion of larvae surviving from hatching to a given life stage. To avoid situations of log(0) values, data were transformed using the formula p´ = [p(N − 1) + 0.5]/N, where N is the number of larvae in the respective replicate (= 10). The proportion of survivors was then transformed into logarithmic and logistic scales. Duration of development was defined as the time elapsed from hatching until the next stage (e.g. duration of development to the megalopa). Body mass (B) was determined as dry mass (DW), and carbon (C) and nitrogen content (N) in freshly hatched zoea I and freshly moulted megalopa. The instantaneous growth rate was calculated as log(BM/BZI)/D, where BZI is the body mass parameter (DW, C, or N) of the freshly hatched zoea I, BM is the body mass parameter of the megalopa, and D the duration of development to the megalopa.
The experimental design for larval rearing was factorial with 3 fixed and orthogonal factors: population (P), salinity (S), and temperature (T). Female of origin (F) was nested in the interaction of salinity and population, as a random factor. Statistical analysis was carried out in R (version 4.2.2) using a backwards model selection approach (Zuur et al. 2009) based on generalised least squares. We used the packages “nlme” (Pinheiro et al. 2019), and the functions “lme” and “gls”. Model selection took place in two steps. First, models for random terms were fitted with restricted maximum likelihood (REML). Second, the model with the best random structure was refitted using maximum likelihood (ML), and selection was carried out of the fixed structure. In both cases, model selection was based on the corrected Akaike information criterion (AICc).
For survival, the best models retained all 2-way interactions (see “Results”). For the subsequent exploration of effects, we fitted quadratic models to the survival data instead of performing multiple comparisons using a post-hoc test. In particular, we used polynomial models to estimate the temperature and salinity at which survival was maximized at each population.
For duration of development, dry mass, elemental composition, and growth we needed to perform multiple separate analysis strains as not all larvae metamorphosed to megalopa at all factor combinations (e.g. we had low survival in Baltic populations) and the design became disconnected (Table 1). For duration of development, four separate statistical analysis were carried out as follows: (1) focus on three salinities (20, 25, and 33 PSU), two temperatures (21 and 24 °C) and three populations (Helgoland, Sylt, and Kiel). (2) Focus on all populations but at one salinity (25 PSU) and two temperatures (21 and 24 °C). (3) focus on three salinities (20, 25, and 33 PSU), three temperatures (18, 21, and 24 °C) but only for Helgoland and Sylt populations; (4) Including all temperatures (> 15 °C), but only one salinity (33 PSU) and the populations from Helgoland, Sylt, and Kiel. Likewise, four separate analyses were conducted for body mass and growth: (1) With focus on two salinities (20 and 25 PSU), two temperatures (21 and 24 °C), and three populations (Helgoland, Sylt, and Kiel). (2) Focus on two salinities (25 and 33 PSU), three temperatures (18, 21, and 24 °C), and two populations (Sylt and Helgoland). (3) Including all populations but at only one salinity (25 PSU) and temperature (24 °C). (4) Including all populations but only one combination of salinity (20 PSU) and temperature (21 °C).
To assess genetic differentiation, the COI sequences were visually inspected for sequencing mistakes, assembled, aligned, and trimmed using the bioinformatics software Geneious Prime (Ver.2020.0.3 Kearse et al. 2012). All subsequent population genetic analysis were conducted in the R environment (R version 4.2.2; 2022). Haplotype network was produced using the R packages ggplot2 (Wickham 2016), scatterpie (Guangchuang 2022), and rworldmap (South 2011). Population differentiation was calculated using Jost´s D and PhiST with the packages “adegenet” (Jombart 2008) and statistical parsimony (Templeton et al. 1992) in “pegas” (Paradis 2010) followed by bootstrapping with 1000 replicates.
Results
For simplicity, we use the term “North Sea populations” for larvae obtained from Sylt and Helgoland and “Baltic Sea populations” for those obtained from Kiel and Neustadt. However, we want to emphasise that we do not claim that these animals represent the whole respective seas. Likewise, we refer to the animals from one sampling site as local populations without inferring that these “populations” are separated or distinctive in terms of connectivity or genetics. Readers are advised to exercise caution and refrain from making unwarranted generalizations based on these labels, as the representativeness of the samples may not extend to the broader characteristics of the North and Baltic Seas or the specific localities in question.
Larval survival, duration of development, and growth rates
Larvae succesfully developed to megalopa at temperatures > 15 °C and salinities > 10 PSU. However, larval survival varied considerably among populations and it was contingent on temperature-salinity combinations (Fig. 2); best statistical models retained interactions between population, temperature, and salinity (Table S1). At 15 °C, larvae failed to reach the megalopa stage at all salinity treatments, irrespective of female of origin or population. Low survival at 15 °C was found for larvae hatching from most females, already at the zoea II, especially at 10 PSU (Fig. S5). Additionally, at 10 PSU, there were no survivors to zoea III, irrespective of female of origin, population, and temperature (Fig. S5). Only in the temperatures ≥ 18 °C and salinities ≥ 15 PSU successful development to megalopa occurred. Larvae from North Sea populations reared in the salinity range 20–33 PSU showed an increasing trend in survival towards the highest temperatures (Fig. 2), with quadratic models indicating a maximum survival at the maximum temperature tested, 24 °C (Fig. 3). This pattern was already observed at the zoea II and then exacerbated in the late zoeal stages (Fig. S5). By contrast, for all studied populations, survival was consistently low at 10 and 15 PSU, irrespective of temperature, and at 15 °C irrespective of salinity.
Hemigrapsus takanoi. Average survival from hatching to megalopa as a response to temperature and salinity discriminated by population. Comparison between populations from the North Sea: Helgoland and Sylt (left panels) and the Baltic Sea: Kiel and Neustadt (right panels) by temperature (15, 18, 21, 24 °C) and salinity (33 PSU, orange diamonds; 25 PSU, purple upwards triangles; 20 PSU, green circles; 15 PSU, blue squares, 10 PSU, red downwards triangles). For the North Sea populations, the habitat/embryonic salinity was 33 PSU, for Kiel 15 PSU and for Neustadt 10 PSU. Data shown as means ± SE for larvae produced by each female from each population (Helgoland: 3; Sylt: 4; Kiel: 3; Neustadt: 3)
Quadratic polynomial models fitted to survival to the megalopa in response to temperature (°C) for different combinations of salinity and population. Models with significant trends for quadratic terms are marked with a red arrow and label indicating the temperature of highest predicted survival by salinity and population. Blue points indicate mean observed survival by female. No predictions could be made for the Neustadt population due to the low number of survivors and therefore high number of zeros in the data. For the North Sea populations, the habitat/embryonic salinity was 33 PSU, for Kiel 15 PSU and for Neustadt 10 PSU
When reared in the salinity range 20 – 33 PSU, larvae from the Baltic Sea populations had reduced survival (Fig. 2) compared to the North Sea populations. Importantly, in the Baltic populations, larval survival was consistently low at salinities 10 and 15 PSU, which are within the salinity range experienced by adults in the natural habitat (i.e., SW Baltic Sea). Except for larvae from Kiel in the 33 PSU treatment, larval survival increased with temperature. Within the Baltic Sea, the Kiel population showed a fairly strong and positive response to high temperatures (21 and 24 °C) as compared to those of Neustadt (very low survival, < 10%), but weaker than the one exhibited by the North Sea populations (> 25%). For the Kiel population, survival of larvae reared at 21 and 24 °C, had an estimated maximum at a slightly lower salinity (Kiel 24–25.5 PSU vs. Sylt & Helgoland 25.5–27.5 PSU) than those of the North Sea populations (Fig. 4); the difference between those salinities was small (~ 1 PSU difference between Kiel and Helgoland) and the survival curve was flat, showing that survival varied little within that range. In addition, the estimated salinity giving the maximum survival in Kiel (24–25 PSU) was much higher than the salinity surrounding the local population (~ 15 PSU). Larvae from Neustadt showed lower survival than those from the Kiel population already from zoea II (Fig. S5).
Quadratic polynomial models fitted to survival in response to salinity (PSU) for different combinations of temperature and population. Models with significant trends for quadratic terms are marked with a red arrow and label indicating the salinity of highest predicted survival by temperature and population. Blue points indicate mean observed survival by female. No predictions could be made for the Neustadt population due to the low number of survivors and therefore high number of zeros in the data. For the North Sea populations, the habitat/embryonic salinity was 33 PSU, for Kiel 15 PSU and for Neustadt 10 PSU
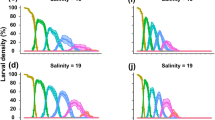
In the North Sea populations there was an important variation in survival among larvae from different females at conditions where survival was high (salinities ≥ 15 PSU, and temperatures ≥ 18 °C); some females produced larvae showing high survival at almost all conditions tested. In some treatments, a small percentage of larvae (< 20%), developed through an additional zoea VI before metamorphosing to megalopa (Fig. S6, Table S2).
Average dry mass (DW), carbon (C) and nitrogen (N) content of freshly hatched zoea I varied considerably among larvae originating from different females within each population. In general, averages followed a trend (Fig. 5) with higher body mass in larvae from Helgoland and Sylt and lowest in those from Neustadt, although the best models did not retain population as an explanatory variable.
Hemigrapsus takanoi. Average dry mass (a), carbon (b) and nitrogen content (c) of freshly hatched zoea I, from four populations (North Sea: Helgoland and Sylt; Baltic Sea: Kiel and Neustadt). Symbols indicate salinity experienced during embryonic development (reflecting the salinity at the collection site): 33, orange diamonds; 15, blue squares and 10, red triangles. Data shown as means ± SE for larvae produced by each female of each population (Helgoland: 6; Sylt: 6; Kiel: 6; Neustadt: 4). For the North Sea populations, the habitat/embryonic salinity was 33 PSU, for Kiel 15 PSU and for Neustadt 10 PSU
Dry mass (Fig. 6a), carbon and nitrogen content of megalopa (Fig. S8) varied among populations as well as among temperature-salinity combinations. Dry mass increased with temperature and salinity with a maximum at 25 or 33 PSU depending on population (interactions population by salinity, and temperature by salinity were retained in the best models: Table S7). When larvae from the Neustadt population were reared at 24 °C, the dry mass of megalopa appeared to be slightly lower but population was not retained in the best model (Table S7). For carbon and nitrogen content, the additive term was retained in the model (Table S8).
Hemigrapsus takanoi. Dry mass (a), duration of development (b), and instantaneous growth rate in terms of dry mass (c) from hatching to megalopa. Comparison between populations from the North Sea (Helgoland and Sylt) and the Baltic Sea (Kiel and Neustadt) by temperature (18, 21, and 24 °C) and salinity (33 PSU, orange diamonds; 25 PSU, purple upwards triangles; 20 PSU, green circles; 15 PSU, blue squares). Data shown as means ± SE for larvae produced by each female of each population (Helgoland: 3; Sylt: 4; Kiel: 3; Neustadt: 3). Means based on a single value were removed (see Fig. S7 in supplementary materials). For the North Sea populations, the habitat/embryonic salinity was 33 PSU, for Kiel 15 PSU and for Neustadt 10 PSU
Duration of development to megalopa (Fig. 6b) decreased with increasing temperatures (range 18 – 24 °C; no larvae survived at 15 °C), following a nonlinear pattern which varied among populations. The best model retained interactions of salinity by population and temperature as a main factor (Table S6). At 18 °C, larvae from the North Sea populations developed in a shorter time (30-40 days) than those from the Baltic Sea populations (45–55 days). There was no evidence of a consistent effect of salinity on duration of development (tested range: 20–33 PSU; Table S6). For the North Sea populations, duration of development was shorter in seawater than for the Baltic Sea (depending on temperature: 20–37 days for North Sea and 29–50 days for the Baltic Sea). For larvae of the North Sea populations, there were no clear effects of salinity on duration of development. For the Helgoland population, the effect of temperature on duration of development was smaller than for Sylt; in addition, larvae from the Helgoland population developed comparably faster across salinities (especially in the lower temperatures: Fig. 6b). In the Baltic Sea, for larvae of the Kiel population, lower salinity (20 and 25 PSU) caused a reduction in duration of development. This reduction was slightly stronger at 24 °C than at 21 °C but we did not find strong evidence in favour of retaining the 3-way full factorial model (ΔAIC = 3: Table S6).
Instantaneous growth rate to megalopa (in terms of dry mass) increased with temperature for larvae from all populations (Fig. 6c, Table S10). There was no evidence of a consistent effect of salinity or population of origin on instantaneous growth rates; the best model retained the interaction between salinity and population (Table S10) or the three 3-way interaction (Helgoland and Sylt populations). We did not find evidence of survival rates being predictors of growth rates, i.e., populations with higher survival rates did not show higher growth rates.
There was a negative relationship between duration of development and body mass (dry mass, carbon and nitrogen content) of the megalopa (Fig. 7, Table S11): larvae that reached the maximum dry mass (or reserves), did so in the shortest time (i.e., those reared at 24 °C). When reared at lower salinities, larvae did not seem to compensate by extending the duration of development, in order to maintain dry mass; instead, larvae reached metamorphosis with different dry mass but at similar times across salinities (Fig. 8).
Hemigrapsus takanoi. Integrated responses of carbon content and duration of development of megalopae from three populations (Helgoland, Sylt, Kiel) reared at three temperatures and four salinities. Salinities (PSU) are shown by colour (33: orange, 25: violet, 20: green), and temperature (°C) is shown as symbols (24: triangles, 21: circles, 18: squares) and surrounded by ellipses for easier identification. Data from Neustadt are not shown due to the low number of survivors. For the North Sea populations, the habitat/embryonic salinity was 33 PSU, for Kiel 15 PSU
Hemigrapsus takanoi. Map of plotted frequencies of the found haplotypes in the four assessed populations: Helgoland and Sylt (North Sea), and Kiel and Neustadt (Baltic Sea). The size of the circles is relative to the sample size (Helgoland: n = 6; Sylt: n = 24; Kiel: n = 19; Neustadt: n = 19); colours indicate the respective haplotype. The pies for Helgoland, Kiel, and Neustadt are offset from their coordinate to avoid overlap—offset is indicated by the dashed line
Molecular analysis
We found six mitochondrial haplotypes, of which four are shared haplotypes and two are private haplotypes. Two haplotypes (H1 and H2) were present in samples from all four populations, i.e., shared between all populations. The haplotype H3 was found in one animal from Neustadt and one from Sylt and H4 was found in two animals from Neustadt and one from Helgoland, i.e., both were shared between North and Baltic Sea populations. Only two haplotypes were private for one population each (H6: Neustadt; H5: Sylt). No significant differences between the populations were found. Neither the PhiST nor Jost’s D did show significant differentiation among populations (Tables S12–S16).
Discussion
We found that survival and growth responses to temperature and salinity, in larvae of Hemigrapsus takanoi vary substantially among populations of the North and Baltic Seas. Survival was particularly low in larvae hatching from females collected in the south-western Baltic Sea populations (Kiel and Neustadt), where salinities are low. By contrast, larvae hatching from females from the North Sea populations generally exhibited higher survival; with larvae growing at higher rates and achieving higher dry mass at higher temperatures. The observed patterns emphasise the role of temperature and salinity in the dynamics of invasions and the importance of quantifying intraspecific variation in larval performance. This is consistent with findings for other non-native crustacean species: temperatures typical of warm summers leads to higher survival, shorter duration of development, and increased dry mass and in some cases also higher reserves (Giménez et al. 2020; Griffith et al. 2021; Espinosa-Novo et al. 2023). In many invertebrates, high body mass at metamorphosis may be a predictor of post metamorphic performance, with larger individuals performing better (Pechenik 1999; Giménez 2006; Torres et al. 2016). Furthermore, because faster developing larvae would settle earlier in the season, juveniles should experience summer temperatures more frequently which could enhance juvenile growth. Moreover, salinity could play an important role considering the differences in tolerance to low salinities between the non-native crab H. takanoi and the native crab Carcinus maenas (Šargač et al. 2021) that coexist in the benthic habitat We divided the following discussion in two sections: first we discuss the implication and potential drivers of the observed variation in the larval performance among populations; second, we examine in detail the apparent mismatch between the larval phenotype and the environmental conditions in the Baltic Sea, near the distribution limit.
Larval performance: potential drivers and implications
Our results highlight the importance of quantifying variation in performance of larvae from different females within and among populations. Larval performance varied considerably at the higher temperature and salinity combinations, in agreement with other studies on decapod crustaceans (Spitzner et al. 2019; Torres et al. 2020; Espinosa-Novo et al. 2023). Furthermore, disregard of interpopulation variability could lead to wrong estimations of species tolerance as shown in this study by the contrasting patterns between the populations of the North and Baltic Seas which have also been found in the European shore crab (Šargač et al. 2021). When evaluating performance of invasive species, extrapolations based on data obtained in native populations are risky and could be misleading, especially for local populations existing at the distribution limit, where suboptimal conditions experienced by adults might impact larval performance. In addition, our results suggest that range expansions (and invasions) into low salinity habitats are very challenging for species of marine origin, in agreement with previous studies (Ojaveer et al. 2010; Nasrolahi et al. 2012; Paiva et al. 2018).
An important question was whether larvae produced in Baltic Sea populations would show signs consistent with local adaptation to low salinity either through a shift in the optimum or higher degree of euryhalinity than those of the North Sea. Local adaptation would contribute to the formation of self-sustaining populations, which increases connectivity and favours range expansion through source-sink dynamics (Giménez et al. 2020). We observed a slight shift in the optimal salinity between populations from the North Sea and the Baltic Sea (Fig. 3 Kiel vs. North Sea populations) and reduced duration of development at moderately low salinities in Kiel as compared to the North Sea populations. However, those shifts in the responses were contingent on temperatures (≥ 21 °C) that may not (yet) be experienced in the Baltic Sea for sufficiently long periods. The response to salinity was consistent with reports on a native Japanese population (Mingkid et al. 2006) and from a previous study on the population in Kiel (Nour et al. 2021, 2022). In addition, our population genetic analysis showed no evidence of a clear separation between the populations and several shared haplotypes. Although we had a restricted number of samples, our genetic results are consistent with those of Geburzi et al. (2020, 2022), based on a much larger sample size and on nine polymorphic microsatellites. In their study, animals from Neustadt appeared more distinct from the North Sea populations than the Kiel population. Our results based on mitochondrial sequence data do not support this result statistically, although we did find one private haplotype in Neustadt and two haplotypes that were absent from the other investigated site in the Baltic Sea. These may be the signature of multiple introductions at this site. Multiple introductions of H. takanoi in Europe (Markert et al. 2014; Makino et al. 2018) could be an explanation for the surprisingly high diversity and lack of founder effect (Roman and Darling 2007). While multiple introductions are often considered advantageous for the success of non-native populations (i.e. they increase genetic diversity and provide novel substrate for adaptive evolution), the above-mentioned differences in larval performance provide only a very weak evidence consistent with local adaptation. Hence, the fact that H. takanoi was only recorded in the Baltic Sea since 2014 (Geburzi et al. 2015) it would be still early to find heritable physiological shifts as observed in other marine crustaceans establishing populations in low salinity habitats (Lee et al. 2011). Other approaches such as high-throughput sequencing could help us to better resolve the genetic structure of H. takanoi to examine populations spanning the North and Baltic Seas.
Maternal effects might explain the observed responses: for instance, females from Neustadt (producing larvae that performed poorly under any condition) may be more stressed than females from the other sites. The reduced dry mass, and carbon and nitrogen content of freshly hatched larvae, for both Baltic populations as compared to the North Sea populations may be driven by allocation of reserves by females and higher energy losses during embryogenesis. Salinity experienced by females is known to drive dry mass, carbon and nitrogen reserves in eggs and embryos, which instead affect body mass at hatching and larval performance (Giménez and Anger 2001, 2003; González-Ortegón and Giménez 2014; Torres et al. 2020). In addition, low salinities experienced during embryogenesis appear to drive larval performance through acclimation (Giménez and Anger 2003) or stress effects not associated to a reduction in reserves at hatching. For instance, in the European shore crab C. maenas, there is evidence of post-zygotic maternal effects whereby exposure of embryos to low salinity affects negatively the adaptive responses to low salinity (Torres et al. 2020; Šargač et al. 2021).
In synthesis, we did not find clear evidence of local adaption to survive low salinities in larvae of H. takanoi, nor any evidence of population differentiation. Stress effects associated to low salinity during embryonic development could explain lower reserves and poor performance in larvae from the Baltic population, especially from Neustadt.
Phenotype–environmental mismatches
Given the low performance at low salinity, it appears that the larval physiological phenotype does not match the low salinities of the Baltic Sea, except perhaps at temperatures ≥ 21 °C. Hence, a critical point is whether populations of the Baltic Sea are maintained through subsidy from the North Sea or (in addition) through successful larval development in areas of the Baltic Sea, where salinities are higher, for example in deep waters (Corell et al. 2012). An initial assessment can be performed combining our experimental results with field data of temperature and salinity (Fig. 9). For instance, based on our experiments, moderate survival in the Kiel fjord is likely (salinity > 15 PSU) with the caveat that temperatures should be ≥ 21 °C for at least the 20 days needed to complete the zoeal development (see Fig. 6b).
a Graph showing sea surface temperature (SST) (°C) in black dots, sea surface salinity (SSS) in dark blue dots, and sea bottom salinity (SBS) (PSU) in Neustadt in light blue dots. Red triangles (black frame: temperature and blue frame: salinity) indicate in situ measurements during our field collections. b Map of selected locations in the south-western Baltic Sea (between Kiel and Neustadt) and the corresponding annual sea surface salinity (PSU) fluctuations. Grey shadowed areas show summer periods (1st of June until 31st of August). Data obtained from Copernicus "Baltic Sea Physics Analysis and Forecast" (Generated using E.U. Copernicus Marine Service Information; https://doi.org/10.48670/moi-00010)
In the Bay of Neustadt, and the greater Bay of Lübeck, survival until megalopa appears unlikely under the salinities on site (< 15 PSU). Because tolerance to low salinities (< 33 PSU) is restricted to high temperatures, larval success would be possible only if the phenological window of larval development matches the windows of high temperature (in summer) and salinities are > 15 PSU. However, in the Bay of Neustadt, salinities higher than 15 PSU only occur in winter and springtime, when storms force North Sea water masses into the Baltic Sea (Lehmann et al. 2022). Thus, currently there is a mismatch between the larval physiological phenotype and the environmental conditions at Neustadt. We therefore hypothesize that adults of H. takanoi at the distribution limit studied here (Mecklenburg Bight) are part of a (demographic) sink population (Pulliam 1988) or individuals perform ontogenetic migrations. In such a case, subsidies may occur through two nonexclusive scenarios: (1) Adults from populations located at areas of high salinity disperse into areas of low salinity. (2) Larvae or adults are introduced into areas of low salinity by human mediated transport, e.g. in the fouling community of ships and boat hulls. Alternatively, such populations are sustained through a third mechanism: (3) Export strategy (Queiroga and Blanton 2004): i.e., early larval stages migrate to (or females release larvae in) areas, characterized by higher salinity, where larvae develop to the megalopa or juvenile stage, which then recolonize areas of low salinity. Such ontogenetic migration could occur between Neustadt and e.g. the Fehmarn Belt area (Fig. 9) where salinities are higher. Larval transport would depend on currents (e.g. surface currents from Neustadt to Fehmarn Belt: Mittelstaedt 2003); zoea I from H. takanoi are known to migrate from near-shore hatching sites to more open waters (e.g. in the Kiel fjord: Geburzi 2018). The elucidation of the actual mechanisms requires field studies quantifying abundance of H. takanoi larvae along the SW Baltic Sea, through e.g. plankton samples or the use of settlement traps at selected coastal sites. For example, a study of the spatial and temporal distribution of H. takanoi larvae is needed to determine if there are ontogenetic migrations; we should observe a progressive change in the spatial distribution of the different larval stages overtime. Additionally, the deployment of settlement traps would garner insights into the presence of megalopa in areas exhibiting lower salinity levels. Furthermore, a systematic field survey targeting the presence of adult specimens could shed light on the spatial distribution of H. takanoi throughout the SW Baltic Sea and the likelihood of connectivity among nearby populations.
Conclusions
In synthesis, our study shows a strong gradient, from the North Sea to the SW Baltic Sea, in the capacity of H. takanoi larvae to develop, and a general inability to survive until metamorphosis in areas where salinity is lower than 15 PSU. Surviving individuals at low salinities showed depressed growth and reduced body mass at metamorphosis, which is likely to compromise post-metamorphic survival. There was only weak evidence of increased tolerance to low salinities in the Baltic populations and no apparent genetic differentiation among the studied populations. Those patterns could be underpinned by constant subsidy from the North Sea that might hinder the establishment of locally adapted larvae in the Baltic Sea. Based on our experiments, survival of larvae to megalopa is unlikely at the current summer salinities experienced at the population limit within the Baltic Sea. Therefore, for populations to persist near the range limit, subsidies or complex ontogenetic migrations are needed. A monitoring programme based, for example on sampling planktonic larvae, will be central to determine if populations are maintained by a ontogenetic migrations or through alternative mechanisms.
Data availability
All data for this paper will be available from PANGAEA ®Data Publisher https://www.pangaea.de/.
References
Anger K, Harms J (1990) Elemental (CHN) and proximate biochemical composition and decapod crustacean larvae. CBPB 97:69–80. https://doi.org/10.1016/0305-0491(90)90180-2
Applebaum SL, Pan T-CF, Hedgecock D, Manahan DT (2014) Separating the nature and nurture of the allocation of energy in response to global change. ICB 54:284–295. https://doi.org/10.1093/icb/icu062
Armsworth PR (2002) Recruitment limitation, population regulation, and larval connectivity in reef fish metapopulations. Ecology 83:1092–1104. https://doi.org/10.1890/0012-9658(2002)083[1092:RLPRAL]2.0.CO;2
Baldanzi S, Storch D, Navarrete SA et al (2018) Latitudinal variation in maternal investment traits of the kelp crab Taliepus dentatus along the coast of Chile. Mar Biol 165:37. https://doi.org/10.1007/s00227-018-3294-2
Belkin IM (2009) Rapid warming of Large Marine Ecosystems. Prog Oceanogr 81:207–213. https://doi.org/10.1016/j.pocean.2009.04.011
Bellard C, Cassey P, Blackburn TM (2016) Alien species as a driver of recent extinctions. Biol Lett 12:20150623. https://doi.org/10.1098/rsbl.2015.0623
Boersma M, Grüner N, Tasso Signorelli N et al (2016) Projecting effects of climate change on marine systems: is the mean all that matters? Proc R Soc B 283:20152274. https://doi.org/10.1098/rspb.2015.2274
Boyd PW, Collins S, Dupont S et al (2018) Experimental strategies to assess the biological ramifications of multiple drivers of global ocean change-A review. Glob Change Biol 24:2239–2261. https://doi.org/10.1111/gcb.14102
Burrows MT, Bates AE, Costello MJ et al (2019) Ocean community warming responses explained by thermal affinities and temperature gradients. Nat Clim Chang 9:959–963. https://doi.org/10.1038/s41558-019-0631-5
Caley MJ, Carr MH, Hixon MA et al (1996) SRecruitment and the local dynamics of open marine populations. Annu Rev Ecol Syst 27:477–500. https://doi.org/10.1146/annurev.ecolsys.27.1.477
Chan FT, Briski E (2017) An overview of recent research in marine biological invasions. Mar Biol 164:121. https://doi.org/10.1007/s00227-017-3155-4
Corell H, Moksnes P, Engqvist A et al (2012) Depth distribution of larvae critically affects their dispersal and the efficiency of marine protected areas. Mar Ecol Prog Ser 467:29–46. https://doi.org/10.3354/meps09963
Cowen RK, Paris CB, Srinivasan A (2006) Scaling of connectivity in marine populations. Science 311:522–527. https://doi.org/10.1126/science.1122039
Dauphinais JD, Miller LM, Swanson RG, Sorensen PW (2018) Source–sink dynamics explain the distribution and persistence of an invasive population of common carp across a model Midwestern watershed. Biol Invas 20:1961–1976. https://doi.org/10.1007/s10530-018-1670-y
Dawirs RR (1986) Influence of limited food supply on growth and elemental composition (C, N, H) of Carcinus maenas (Decapoda) larvae, reared in the laboratory. Mar Ecol Prog Ser 31:301–308. https://doi.org/10.3354/meps031301
Dawirs RR, Püschel C, Schorn F (1986) Temperature and growth in Carcinus maenas L. (Decapoda: Portunidae) larvae reared in the laboratory from hatching through metamorphosis. J Exp Mar Bio Ecol 100:47–74
de Amorim F, de LL, Wiltshire KH, Lemke P, et al (2023) Investigation of marine temperature changes across temporal and spatial Gradients: Providing a fundament for studies on the effects of warming on marine ecosystem function and biodiversity. Prog Oceanogr 216:103080. https://doi.org/10.1016/j.pocean.2023.103080
Duarte C (2007) Marine ecology warms up to theory. Trends Ecol Evol 22:331–333. https://doi.org/10.1016/j.tree.2007.04.001
Durrant HMS, Clark GF, Dworjanyn SA et al (2013) Seasonal variation in the effects of ocean warming and acidification on a native bryozoan, Celleporaria nodulosa. Mar Biol 160:1903–1911. https://doi.org/10.1007/s00227-012-2008-4
Espinosa-Novo N, Giménez L, Boersma M, Torres G (2023) On their way to the north: larval performance of Hemigrapsus sanguineus invasive to the European coast—a comparison with the native European population of Carcinus maenas. Biol Invas. https://doi.org/10.1007/s10530-023-03095-3
Folmer O, Black M, Hoeh W, et al (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3
Gallardo B, Clavero M, Sánchez MI, Vilà M (2016) Global ecological impacts of invasive species in aquatic ecosystems. Glob Change Biol 22:151–163. https://doi.org/10.1111/gcb.13004
García Molinos J, Halpern BS, Schoeman DS et al (2016) Climate velocity and the future global redistribution of marine biodiversity. Nature Clim Change 6:83–88. https://doi.org/10.1038/nclimate2769
GBIF (2022) GBIF.org (03 August 2022) GBIF Hemigrapsus takanoi Occurrence Download. https://doi.org/10.15468/dl.xshtwy
Geburzi JC (2018) New species from the Pacific: establishment and dispersal of two invasive crabs (genus Hemigrapsus) in German coastal waters. Dissertation, Christian-Albrechts-Universität zu Kiel
Geburzi J, Graumann G, Köhnk S, Brandis D (2015) First record of the Asian crab Hemigrapsus takanoi Asakura & Watanabe, 2005 (Decapoda, Brachyura, Varunidae) in the Baltic Sea. BIR 4:103–107. https://doi.org/10.3391/bir.2015.4.2.06
Geburzi JC, Ewers-Saucedo C, Brandis D, Hartl GB (2020) Complex patterns of secondary spread without loss of genetic diversity in invasive populations of the Asian shore crab Hemigrapsus takanoi (Decapoda) along European coasts. Mar Biol 167:180. https://doi.org/10.1007/s00227-020-03790-y
Geburzi JC, Heuer N, Homberger L et al (2022) An environmental gradient dominates ecological and genetic differentiation of marine invertebrates between the North and Baltic Sea. Ecol Evol. https://doi.org/10.1002/ece3.8868
Giménez L (2006) Phenotypic links in complex life cycles: conclusions from studies with decapod crustaceans. ICB 46:615–622. https://doi.org/10.1093/icb/icl010
Giménez L, Anger K (2001) Relationships among salinity, egg size, embryonic development, and larval biomass in the estuarine crab Chasmagnathus granulata Dana, 1851. J Exp Mar Bio Ecol 260:241–257. https://doi.org/10.1016/S0022-0981(01)00258-1
Giménez L, Anger K (2003) Larval performance in an estuarine crab, Chasmagnathus granulata, is a consequence of both larval and embryonic experience. Mar Ecol Prog Ser 249:251–264. https://doi.org/10.3354/meps249251
Giménez L, Exton M, Spitzner F et al (2020) Exploring larval phenology as predictor for range expansion in an invasive species. Ecography 43:1423–1434. https://doi.org/10.1111/ecog.04725
Gollasch S (1999) The asian decapod Hemigrapsus penicillatus (de Haan, 1835) (Grapsidae, Decapoda) introduced in European waters: status quo and future perspective. Helgoländer Meeresunters 52:359–366. https://doi.org/10.1007/BF02908909
González-Ortegón E, Giménez L (2014) Environmentally mediated phenotypic links and performance in larvae of a marine invertebrate. Mar Ecol Prog Ser 502:185–195. https://doi.org/10.3354/meps10708
Gräwe U, Friedland R, Burchard H (2013) The future of the western Baltic Sea: two possible scenarios. Ocean Dyn 63:901–921. https://doi.org/10.1007/s10236-013-0634-0
Griffith K, Jenkins SR, Giménez L (2021) Larval tolerance to food limitation is stronger in an exotic barnacle than in its native competitor. Zool 145:125891. https://doi.org/10.1016/j.zool.2020.125891
Guangchuang Y (2022) R package “scatterpie” Scatter Pie Plot Version 0.1.8
Gurevitch J, Fox GA, Wardle GM et al (2011) Emergent insights from the synthesis of conceptual frameworks for biological invasions: conceptual frameworks for biological invasions. Ecol Lett 14:407–418. https://doi.org/10.1111/j.1461-0248.2011.01594.x
Haubrock PJ, Cuthbert RN, Sundermann A et al (2021a) Economic costs of invasive species in Germany. NeoBiota 67:225–246. https://doi.org/10.3897/neobiota.67.59502
Haubrock PJ, Turbelin AJ, Cuthbert RN et al (2021b) Economic costs of invasive alien species across Europe. NeoBiota 67:153–190. https://doi.org/10.3897/neobiota.67.58196
Helmuth B, Broitman BR, Blanchette CA et al (2006a) Mosaic patterns of thermal stress in the rocky intertidal zone: implications for climate change. Ecol Monogr 76:461–479. https://doi.org/10.1890/0012-9615(2006)076[0461:MPOTSI]2.0.CO;2
Helmuth B, Mieszkowska N, Moore P, Hawkins SJ (2006b) Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Annu Rev Ecol Evol Syst 37:373–404. https://doi.org/10.1146/annurev.ecolsys.37.091305.110149
Henry M, Leung B, Cuthbert RN et al (2023) Unveiling the hidden economic toll of biological invasions in the European Union. Environ Sci Eur 35:43. https://doi.org/10.1186/s12302-023-00750-3
Hiddink JG, Burrows MT, García Molinos J (2015) Temperature tracking by North Sea benthic invertebrates in response to climate change. Glob Change Biol 21:117–129. https://doi.org/10.1111/gcb.12726
Hofmann GE, Smith JE, Johnson KS et al (2011) High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6:e28983. https://doi.org/10.1371/journal.pone.0028983
Johnston EL, Piola RF, Clark GF (2009) The role of propagule pressure in invasion success. In: Rilov G, Crooks JA (eds) Biological invasions in marine ecosystems. Springer, Berlin, Heidelberg, pp 133–151
Jombart T (2008) adegenet : a R package for the multivariate analysis of genetic markers. Bioinform 24:1403–1405. https://doi.org/10.1093/bioinformatics/btn129
Kearse M, Moir R, Wilson A et al (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinform 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Lee CE, Kiergaard M, Gelembiuk GW et al (2011) Pumping ions: rapid parallel evolution of ionic regulation following habitat invasions: ion-motive ATPase evolution during invasions. Evolution 65:2229–2244. https://doi.org/10.1111/j.1558-5646.2011.01308.x
Lehmann A, Myrberg K, Post P et al (2022) Salinity dynamics of the Baltic Sea. Earth Syst Dynam 13:373–392. https://doi.org/10.5194/esd-13-373-2022
Makino W, Miura O, Kaiser F et al (2018) Evidence of multiple introductions and genetic admixture of the Asian brush-clawed shore crab Hemigrapsus takanoi (Decapoda: Brachyura: Varunidae) along the Northern European coast. Biol Invas 20:825–842. https://doi.org/10.1007/s10530-017-1604-0
Markert A, Raupach MJ, Segelken-Voigt A, Wehrmann A (2014) Molecular identification and morphological characteristics of native and invasive Asian brush-clawed crabs (Crustacea: Brachyura) from Japanese and German coasts: Hemigrapsus penicillatus (De Haan, 1835) versus Hemigrapsus takanoi Asakura & Watanabe 2005. Org Divers Evol 14:369–382. https://doi.org/10.1007/s13127-014-0176-4
Mingkid WM, Yokota M, Seiichi W (2006) Salinity tolerance of larvae in the Penicillate crab Hemigrapsus takanoi (Decapoda: Brachyura: Grapsidae). La Mer 44:17–21
Mittelstaedt E (2003) Nord- und Ostsee: Gezeiten, Strömungen, Wasserschichtung. 2-Relief, Boden, Wasser, pp 118–119
Nasrolahi A, Pansch C, Lenz M, Wahl M (2012) Being young in a changing world: how temperature and salinity changes interactively modify the performance of larval stages of the barnacle Amphibalanus improvisus. Mar Biol 159:331–340. https://doi.org/10.1007/s00227-011-1811-7
Noël PY, Tardy E, d’Acoz d’Udekem C (1997) Will the crab Hemigrapsus penicillatus invade the coasts of Europe? C R Acad Sci III. https://doi.org/10.1016/S0764-4469(97)84823-8
Nour O, Stumpp M, Morón Lugo SC et al (2020) Population structure of the recent invader Hemigrapsus takanoi and prey size selection on Baltic Sea mussels. Aquat Invas 15:297–317. https://doi.org/10.3391/ai.2020.15.2.06
Nour O, Pansch C, Lenz M et al (2021) Impaired larval development at low salinities could limit the spread of the non-native crab Hemigrapsus takanoi in the Baltic Sea. Aquat Biol 30:85–99. https://doi.org/10.3354/ab00743
Nour OM, Pansch C, Stumpp M (2022) Freshening and warming may restrict dispersal of Hemigrapsus takanoi into the Baltic Proper due to interactive effects on larval survival and feeding. Mar Biol 169:125. https://doi.org/10.1007/s00227-022-04112-0
Ojaveer H, Jaanus A, MacKenzie BR et al (2010) Status of Biodiversity in the Baltic Sea. PLoS ONE 5:e12467. https://doi.org/10.1371/journal.pone.0012467
Paiva F, Barco A, Chen Y et al (2018) Is salinity an obstacle for biological invasions? Glob Chang Biol 24:2708–2720. https://doi.org/10.1111/gcb.14049
Pandori LLM, Sorte CJB (2019) The weakest link: sensitivity to climate extremes across life stages of marine invertebrates. Oikos 128:621–629. https://doi.org/10.1111/oik.05886
Paradis E (2010) pegas: an R package for population genetics with an integrated–modular approach. Bioinform 26:419–420. https://doi.org/10.1093/bioinformatics/btp696
Pechenik J (1999) On the advantages and disadvantages of larval stages in benthic marine invertebrate life cycles. Mar Ecol Prog Ser 177:269–297. https://doi.org/10.3354/meps177269
Pinheiro J, Bates D, DebRoy S, Sarkar D (2019) nlme: linear and nonlinear mixed effects models. R-package version 3.1–140
Poloczanska ES, Brown CJ, Sydeman WJ et al (2013) Global imprint of climate change on marine life. Nat Clim Change 3:919–925. https://doi.org/10.1038/nclimate1958
Pulliam HR (1988) Sources, sinks, and population regulation. Am Nat 132:652–661. https://doi.org/10.1086/284880
Queiroga H, Blanton J (2004) Interactions between behaviour and physical forcing in the control of horizontal transport of decapod crustacean larvae. In: Advances in Marine Biology. Elsevier, pp 107–214
Reusch TBH, Dierking J, Andersson HC, et al (2018) The Baltic Sea as a time machine for the future coastal ocean. Sci Adv 4:eaar8195. https://doi.org/10.1126/sciadv.aar8195
Robins PE, Skov MW, Lewis MJ et al (2016) Impact of climate change on UK estuaries: a review of past trends and potential projections. Estuar Coast Shelf Sci 169:119–135. https://doi.org/10.1016/j.ecss.2015.12.016
Roman J, Darling J (2007) Paradox lost: genetic diversity and the success of aquatic invasions. Trends Ecol Evol 22:454–464. https://doi.org/10.1016/j.tree.2007.07.002
Sanford E, Holzman SB, Haney RA et al (2006) Larval tolerance, gene flow, and the northern geographic range limit of fiddler crabs. Ecology 87:2882–2894. https://doi.org/10.1890/0012-9658
Šargač Z, Giménez L, Harzsch S et al (2021) Contrasting offspring responses to variation in salinity and temperature among populations of a coastal crab: a maladaptive ecological surprise? Mar Ecol Prog Ser 677:51–65. https://doi.org/10.3354/meps13851
Šargač Z, Giménez L, González-Ortegón E, et al (2022) Quantifying the portfolio of larval responses to salinity and temperature in a coastal-marine invertebrate: a cross population study along the European coast. Mar Biol
Somero GN (2002) Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. ICB 42:780–789. https://doi.org/10.1093/icb/42.4.780
Sorte CJB, Williams SL, Carlton JT (2010) Marine range shifts and species introductions: comparative spread rates and community impacts: Range shifts and non-native species introductions. GEB 19:303–316. https://doi.org/10.1111/j.1466-8238.2009.00519.x
Sorte CJB, Ibáñez I, Blumenthal DM et al (2013) Poised to prosper? A cross-system comparison of climate change effects on native and non-native species performance. Ecol Lett 16:261–270. https://doi.org/10.1111/ele.12017
Sorte CJB, Pandori LLM, Cai S, Davis KA (2018) Predicting persistence in benthic marine species with complex life cycles: linking dispersal dynamics to redistribution potential and thermal tolerance limits. Mar Biol 165:20. https://doi.org/10.1007/s00227-017-3269-8
South A (2011) rworldmap: A New R package for Mapping Global Data. The R Journal 3:35–43
Spitzner F, Giménez L, Meth R et al (2019) Unmasking intraspecific variation in offspring responses to multiple environmental drivers. Mar Biol 166:112. https://doi.org/10.1007/s00227-019-3560-y
Templeton AR, Crandall KA, Sing CF (1992) A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics 132:619–633. https://doi.org/10.1093/genetics/132.2.619
Theurich N, Briski E, Cuthbert RN (2022) Predicting ecological impacts of the invasive brush-clawed shore crab under environmental change. Sci Rep 12:9988. https://doi.org/10.1038/s41598-022-14008-0
Torres G, Giménez L, Pettersen A et al (2016) Persistent and context-dependent effects of the larval feeding environment on post-metamorphic performance through the adult stage. Mar Ecol Prog Ser 545:147–160. https://doi.org/10.3354/meps11586
Torres G, Thomas DN, Whiteley NM et al (2020) Maternal and cohort effects modulate offspring responses to multiple stressors. Proc R Soc B 287:20200492. https://doi.org/10.1098/rspb.2020.0492
Torres G, Melzer RR, Spitzner F et al (2021b) Methods to study organogenesis in decapod crustacean larvae. I. larval rearing, preparation, and fixation. Helgol Mar Res 75:3. https://doi.org/10.1186/s10152-021-00548-x
Torres G, Charmantier G, Wilcockson D, et al (2021a) Physiological basis of interactive responses to temperature and salinity in coastal marine invertebrate: implications for responses to warming. Ecol Evol ece3.7552. https://doi.org/10.1002/ece3.7552
Ware C, Berge J, Jelmert A et al (2016) Biological introduction risks from shipping in a warming Arctic. J Appl Ecol 53:340–349. https://doi.org/10.1111/1365-2664.12566
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis.
Zuur AF, Ieno EN, Walker N et al (2009) Mixed effects models and extensions in ecology with R. Springer, New York, NY
Acknowledgements
We are grateful to Julia Haafke (AWI, Germany) for performing the elemental analysis, Sabrina Hüpperling and Pia Steingrübner (both Universität Greifswald, Germany) for their help collecting crabs and rearing larvae, and Jonas Geburzi (ZMT, Germany & Harvard University, USA) for advice on sampling sites and access. We thank the Institute of Clinical Molecular Biology in Kiel (Germany) for providing Sanger sequencing as supported in part by the DFG Clusters of Excellence “Precision Medicine in Chronic Inflammation" and "ROOTS". We thank T. Naujoks, Dr. D. Langfeldt and Dr. B. Löscher for technical support. We would also like to thank two anonymous reviewers and the editor whose comments and suggestions improved the quality of the work. This study was conducted using E.U. Copernicus Marine Service Information https://doi.org/10.48670/moi-00010. This study is part of JPG and NE-N doctoral dissertations. JPG was supported by the Deutsche Forschungsgemeinschaft (Research Training Group 2010: RESPONSE) at the University of Greifswald, in a collaboration with the Alfred-Wegener-Institute. NE-N (AWI) was supported by the Bundesministerium für Bildung und Forschung (Project MERGE; grant n° FKZ 01DN20002), Germany. DMA was funded by the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska Curie grant agreement (101023801) at the French National Centre for Scientific Research (CNRS).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JPG, NE-N, SH, LG, and GT conceived the experimental design. JPG, GT, CE, and AC collected the ovigerous females. JPG performed the experiments. JPG, NE-N, CE, DMA, and GT generated the data for the population genetics analyses. JPG, NE-N, CE, and LG analysed the data. JPG wrote the first draft. All authors contributed to the writing of the manuscript and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Human or animal rights
The research presented in this paper complies with European laws (guidelines from the directives 2010/63/EU of the European parliament and of the Council of 22nd September 2010) on the protection of animals used for scientific purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Geißel, J.P., Espinosa-Novo, N., Giménez, L. et al. Interactive responses to temperature and salinity in larvae of the Asian brush-clawed crab Hemigrapsus takanoi: relevance for range expansion into the Baltic Sea, in the context of climate change. Biol Invasions (2024). https://doi.org/10.1007/s10530-024-03279-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10530-024-03279-5