Abstract
Since the 1960s, clomiphene citrate is used for ovulation induction. Since nonresponse to clomiphene therapy is still not well understood, interindividual variability of clomiphene metabolism has been considered to be a plausible explanation. Therefore, a comprehensive, rapid, sensitive, and specific analytical method for the quantification of (E)- and (Z)-isomers of clomiphene and their putative N-desethyl, N,N-didesethyl, 4-hydroxy, and 4-hydroxy-N-desethyl metabolites, and the N-oxides in human plasma has been newly developed, using HPLC-tandem mass spectrometry and stable isotope-labeled internal standards. All standards other than the parent drug were synthesized in our laboratory. Following protein precipitation analytes were separated on a ZORBAX Eclipse plus C18 1.8 μm column with a gradient of 0.1% formic acid in water and 0.1% formic acid in acetonitrile and detected on a triple quadrupole mass spectrometer with positive electrospray ionization in the multiple reaction monitoring mode. Lower limit of quantification for metabolites ranged from 0.06 ng/mL for clomiphene-N-oxides to 0.3 ng/mL for (E)-N-desethylclomiphene. The assay was validated according to FDA guidelines. Plasma levels of clomiphene and its metabolites were measured in two women after single-dose treatment with clomiphene.

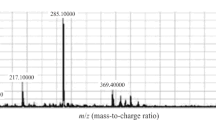
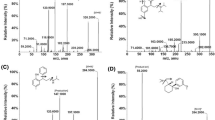
Structures of clomiphene isomers and overlay of MRM-chromatograms of clomiphene and its metabolites






Similar content being viewed by others
References
Dewailly D, Hieronimus S, Mirakian P, Hugues J (2010) Ann Endocrinol 71:8–13
Szutu M, Morgan DJ, McLeish M, Phillipou G, Blackman GL, Cox LW, Dollman W (1989) Br J Clin Pharmacol 27:639–640
Baustian CL, Mikkelson TJ (1986) J Pharm Biomed Anal 4:237–246
Urmös I, Benkö SM, Klebovich I (1993) J Chromatogr 617:168–172
Harman PJ, Blackman GL, Phillipou G (1981) J Chromatogr 225:131–138
Liu W, Zhang L, Chen S, Duan H, Chen X, Wei Z, Chen G (2009) Anal Chim Acta 631:47–53
Crewe HK, Ghobadi C, Gregory A, Rostami-Hodjegan A, Lennard MS (2007) J Chromatogr B Analyt Technol Biomed Life Sci 847:296–299
Liu X, Zhang J, Yin J, Duan H, Wu Y, Shao B (2010) Anal Bioanal Chem 396:2977–2985
Brauch H, Mürdter TE, Eichelbaum M, Schwab M (2009) Clin Chem 55:1770–1782
Jaremko M, Kasai Y, Barginear MF, Raptis G, Desnick RJ, Yu C (2010) Anal Chem 82:10186–10193
Ghobadi C, Gregory A, Crewe HK, Rostami-Hodjegan A, Lennard MS (2008) Drug Metab Pharmacokinet 23:101–105
Ghobadi C, Mirhosseini N, Shiran MR, Moghadamnia A, Lennard MS, Ledger WL, Rostami-Hodjegan A (2009) J Clin Pharmacol 49:147–154
Lucas AN, Tanol M, McIntosh MP, Rajewski RA (2006) Synth Commun 36:3371
ter Wiel MKJ, van Delden RA, Meetsma A, Feringa BL (2003) J Am Chem Soc 125:15076–15086
Bari SS, Bose AK, Chaudhary AG, Manhas MS, Raju VS, Robb EW (1992) J Chem Educ 69:938
Bose AK, Jayaraman M, Okawa A, Bari SS, Robb EW, Manhas MS (1996) Tetrahedron Lett 37:6989–6992
Palopoli FP, Feil VJ, Allen RE, Holtkamp DE, Richardson A (1967) J Med Chem 10:84–86
Guidance for Industry—bioanalytical method validation (2001) U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), Rockville, MD, USA. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf. Accessed on 31 Jan 2011
Ghobadi C, Amer S, Lashen H, Lennard MS, Ledger WL, Rostami-Hodjegan A (2009) Fertil Steril 91:1135–1140
Katzenellenbogen BS, Norman MJ, Eckert RL, Peltz SW, Mangel WF (1984) Cancer Res 44:112–119
Substances and Methods Prohibited at All Times (2011) World Anti-Doping Agency, Montreal (Quebec). http://www.wada-ama.org/en/World-Anti-Doping-Program/Sports-and-Anti-Doping-Organizations/International-Standards/Prohibited-List/The-2011-Prohibited-List/Prohibited-at-All-Times/. Accessed 31 Jan 2011
Mazzarino M, Fiacco I, de la Torre X, Botrè F (2008) Eur J Mass Spectrom 14:171–180
Brito DM, de Oca Porto RM, Vidal MTC, Ojeda RS, Pérez AR (2010) J Braz Chem Soc 21:2220–2225
Mareck-Engelke U, Sigmund G, Opfermann G, Geyer H, Schänzer W (2001) In: Schänzer W, Geyer H, Gotzmann A, Mareck-Engelke U (eds) Recent advances in doping analysis (9). Sport und Buch Strauß, Köln
Acknowledgments
We are grateful to Svitlana Igel and Gabriele Böhmer for supervising the clinical trial. This work was supported by grants from the Robert Bosch Foundation (Stuttgart, Germany) and from the Federal Ministry for Education and Research (BMBF, Berlin, Germany; 03IS2061C and 01ZP0502).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ganchev, B., Heinkele, G., Kerb, R. et al. Quantification of clomiphene metabolite isomers in human plasma by rapid-resolution liquid chromatography–electrospray ionization–tandem mass spectrometry. Anal Bioanal Chem 400, 3429–3441 (2011). https://doi.org/10.1007/s00216-011-5045-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5045-9