Abstract
Anthracycline chemotherapy is commonly used to treat breast cancer yet may increase the level of matrix metalloproteinases (MMP) -2 and -9, which increase the risk of atherosclerosis. While exercise has been shown to reduce the level of MMP in patients with diabetes, high intensity interval training (HIIT) has not been utilized to improve level of MMP in women with breast cancer receiving anthracycline chemotherapy. Thirty women were randomized to either 8-week HIIT or control (CON) group. The CON group was offered the HIIT intervention after 8 weeks. MMP-1, -2 -7, -9, tissue inhibitor of MMP (TIMP) -1, and-2 were measured at baseline and post-intervention. Repeated measures ANCOVA and paired t-test were performed to assess changes in MMP and TIMP. Post-intervention, no significant between-group differences were observed for MMP and TIMP. However, within-group decrease in MMP-9 was observed in the HIIT group [104.3(51.9) to 65.2(69.1); P = 0.01]. MMP-9 in the CON group was not significantly changed [115.5(47.2) to 90.4(67.9);]. MMP-2 significantly increased in both the HIIT group [76.6(11.2) to 83.2(13.1); P = 0.007) and the CON group [69.0(8.9) to 77.6(11.1) P = 0.003). It is unclear whether an 8-week HIIT intervention influences MMP-9 in breast cancer patients undergoing anthracycline chemotherapy. Additional investigations are required to understand the exercise-induced changes in MMP-2 and -9 in women undergoing anthracycline chemotherapy.
Similar content being viewed by others
Introduction
Anthracycline-based chemotherapy is one of the most commonly used neoadjuvant or adjuvant chemotherapies for the treatment of breast cancer, yet its clinical use has been associated with cardiovascular toxicity, most notably as a decrease in left ventricular ejection fraction more than 10% or to cutoff value of 53%1,2. The cardiovascular toxicity of anthracycline-based chemotherapy is associated with oxidative stress which degrades the extracellular matrix (ECM) structure within the vascular system3. The ECM surrounding vascular endothelial cells, also known as endothelial ECM, is primarily regulated by the enzymes matrix metalloproteinases (MMP) and tissue inhibitor of MMPs (TIMP)4,5. The overexpression of certain MMPs (i.e., MMP-2 and -9) is correlated with increased coronary plaque (r = 0.49, P = 0.007) in patients with coronary artery disease6. MMP-2 has been reported higher in patients with metabolic syndrome compared to healthy controls (3,452 ± 429 vs 4,263 ± 427)7. MMP-9 was significantly associated with high blood pressure [beta: 2.9 (0.4–5.4), low HDL [-2.5 (-5.0–0.0), triglycerides (best 3.7 (1.4–6.1) and overall cardiovascular risk [4.7 (2.6–6.8) after adjusting for age, and sex8. Further, increased levels of MMP-9 promote plaque development in the vasculature9, and elevated levels of MMP-9 has been found in patients with hypertension than normotensive controls (2.31 ± 0.2 vs 1.94 ± 0.3)10 and correlated with higher femoral atherosclerosis (r = 0.16; P < 0.01)11. Moreover, anthracycline-based chemotherapy increases oxidative stress, measured by the level of reactive oxygen species, which causes the overexpression MMP-2 and MMP-912,13,14. The adverse nature of anthracycline-induced alterations in ECM-regulating enzymes provides a strong rationale for the design and testing of intervention strategies (e.g. exercise) to improve vascular endothelial function, and ultimately reduce chemotherapy-related cardiovascular mortality in breast cancer patients15,16.
MMP-2 and -9 are reduced with exercise interventions in patients with type 2 diabetes17 and breast cancer18; other MMPs such as MMP-1, -7, -9, TIMP-1 and -2, have not been determined whether exercise improves the level of MMPs in clinical populations such as type 2 diabetes. While there is evidence showing that moderate-intensity aerobic exercise training may decrease MMP-2 and -9 levels in clinical research settings17,18, the effects of high intensity interval training (HIIT) on MMP levels have not been investigated in any population. HIIT is an exercise strategy that maximizes exercise intensity by using bursts of concentrated effort alternated with recovery periods19. HIIT allows patients to perform vigorous intensity exercise due to the ‘on-off’ pattern of the activity. The “on” portion of HIIT typically involves 1–4 minutes performed at 80–90% of peak power output (PPO), followed by the “off” period (2–3 minutes of active break [10% PPO])20. This interval-based strategy has been shown to be more effective than moderate continuous intensity aerobic exercise for improving endothelial function among individuals with stroke or coronary artery disease21,22,23, and reduces liver fat and increases early diastolic filling rate in patients with non-alcoholic fatty liver disease24.
The aim of this pilot study was to determine the effects of an 8-week HIIT intervention on ECM-regulating enzymes in early stage breast cancer patients receiving anthracycline-based chemotherapy. We also explored the comparative changes in other MMP and TIMP, including MMP-1, -7, -10, and TIMP-1 and -2. We hypothesized that a group of participants that performed the 8-week HIIT intervention would demonstrate greater decreases in circulating serum MMP-2 and -9 compared to a group of participants that did not perform HIIT (CON).
Methods
Detailed methods have been published25. In brief, this pilot study compared HIIT group versus CON group on baseline to post-intervention changes in ECM-regulating enzymes. Participants were recruited from the breast cancer clinics at the University of Southern California (USC) Norris Comprehensive Cancer Center (NCCC) and the Los Angeles County Medical Center. Following written informed consent, eligible participants completed baseline tests (week 0) within one week prior to the start of the intervention. Participants were randomly assigned by computer-generated, investigator-blinded randomization assignments; randomization was stratified by neoadjuvant versus adjuvant anthracyclines treatment in a 1:1 allocation ratio by the Clinical Investigation Support Office at the NCCC. Participants randomized to the HIIT group completed the exercise sessions for a total of 90 min of weekly exercise during the 8-week intervention period. Participants randomized to the CON group were asked to maintain their current level of physical activity, which was defined as less than 30 min of total exercise per week. All participants returned within 1 week following completion of the 8-week study period for post-intervention (week 9) testing. For ethical reasons related to the overall beneficial effect of exercise and to encourage participation, participants in the CON group were offered the same HIIT intervention following the 8-week study period.
Study population
Participants were recruited if women were over 18 years of age diagnosed stage I-III breast cancer; planned to initiate neoadjuvant or adjuvant anthracycline chemotherapy within 1 week of the start of anthracycline-based chemotherapy. In addition, participants were currently participating in less than 30 minutes of physical activity per week, were not smoking during previous 12 months, and willing to travel to the exercise facility at USC. Physician clearance was provided to participate in the exercise program. We excluded participants if women had a history of chronic disease including diabetes, uncontrolled hypertension or thyroid disease; weight reduction >10% within past 6 months; metastatic disease; overt cardiovascular diseases (myocardial infarction, stroke, angina). Further contra-indications to exercise or participation in regular exercise were confirmed to exclude potential participants. Recruitment period was between August 15th, 2017 and August 6th, 2018. The protocol and informed consent were IRB-approved (HS-15-00227) and registered (ClinicalTrials.gov: NCT02454777; date of registration: May 27th, 2015). All methods were carried out in accordance with relevant guidelines and regulations along with the approval and informed consent.
Exercise intervention
Participants assigned to the HIIT group were asked to perform supervised one-on-one exercise sessions 3 times/week using a stationary bicycle (Life Fitness 95 Elevation Series, Rosemont, IL). Prior to initiating the exercise intervention, relative exercise intensity was individually prescribed for each HIIT participant based on peak power output (PPO; watts) performed at 60 rpm, which was measured by a VO2max fitness test performed on a stationary bike23. PPO was defined as the highest power output generated during a maximal cycling test22. Each exercise session consisted of a 5-minute warm-up performed at 10% PPO, followed by a 20-minute HIIT protocol. Based on previous studies that reported significant improvements of cardiovascular function with high compliance to the HIIT intervention22,23, the HIIT protocol consisted of 7 bouts of 1 minute high-intensity exercise (90% of PPO) followed by 2 minutes of low intensity (10% of PPO). Participants were asked to rest at least 24 hours before the next session and to complete their sessions on days when they did not receive chemotherapy infusions. We documented power output, heart rate, rating of perceived exertion (RPE; rated on the Borg scale of 6–20), and total minutes of exercise for each session. Participants were encouraged to make up any missed sessions within the same week. The CON group was asked to maintain their level of physical activity during the 8-week study period. Following the 8-weeks of intervention, we offered the same HIIT intervention to the CON group.
Outcome measures
MMP and TIMP
A fasting venous blood sample was obtained from each participant by a licensed phlebotomist. Blood samples were centrifuged at 3,000 rpm for 10 minutes, and the serum portion was pipetted into 0.5 mL aliquots using a sterile pipette. Aliquots were stored at −80 °C until further processing. Participants in the HIIT group were requested to avoid any strenuous physical activities for at least 48 h before blood sampling at baseline (week 0) and post-intervention (week 9). MMP-1, -2, -7, -9, and -10 concentrations were examined as a single batch at study completion and processed using the magplex suspension bead array immuno-assays on a Luminex 100 Bioanalyzer (Luminex 100, Luminex Corporation, Austin, Texas) according to the kit manufacturer’s instructions (Milliplex ELISA kits, Millipore, MA) by the Metabolism Core Laboratory/Human Physiology laboratory of the University of Alabama Birmingham. Specifically, 50 ml of each serum sample were incubated with fluorokine-coloured microspheres coated with specific antibodies, and analytes were allowed to bind to the specific antibody-coated microspheres. Samples were then washed and incubated with biotinylated antibodies and phycoerythrin-conjugated streptavidin. Finally, fluorescence was detected using a flow cytometry technique (Luminex 100, Luminex Corporation, Austin, Texas). All samples were assayed in triplicate wells (25 uL per well), and the mean of these results was used for statistical analysis. MMP concentration was calculated by reference to an eight-point spline fit curve.
Statistical analyses
Baseline participant characteristics were summarized by descriptive statistics. Distribution of outcomes were evaluated and presented as mean (SD) for continuous outcomes and frequency (%) for categorical outcomes. Normality was evaluated by the Kolmogorov-Smirnov test. If data were not normally distributed, non-parametric corollary for continuous outcomes and χ2 test for categorical outcomes were used. As there was no influence of outliers, data points were not removed from the original dataset. Group comparisons of baseline participant characteristics were made using t-test or non-parametric corollary for continuous outcomes and χ2 test for categorical outcomes26. Participant baseline characteristics that were different across groups were included as covariates in the statistical analyses. Given the small sample size (N = 30), baseline variables with a difference of P < 0.10 were considered as additional covariates after testing for collinearity. An analysis of covariance (ANCOVA) model adjusting for the baseline value of the outcome was performed with treatment group (HIIT vs CON) and time (baseline/post-intervention) as factors27. For within group difference, the changes in MMP from baseline to week 9 were examined by a paired t-test, with a level of significance set at P < 0.0528. Repeated measures ANCOVA on the trial outcomes was a 2 (group: HIIT, CON) x 2 (time: baseline, post-intervention) analysis. Cohen’s d effect size (d) for the changes in MMP was calculated using the difference in means from baseline and post-intervention and the pooled standard deviations29. All analyses were performed with SPSS (v.22).
Ethics approval and consent to participate
The protocol and informed consent were approved by the University of Southern California Institutional Review Board (HS-1500227).
Results
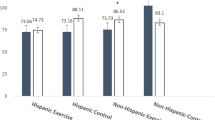
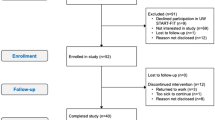
Fifty-eight women were screened for eligibility and 30 participants were enrolled, consented, and randomized to the HIIT (n = 15) or CON groups (n = 15). Baseline characteristics and CONSORT diagram have been published previously30. Briefly, participants were mainly Hispanic white (73%) and BMI was 31.0 ± 7.5 kg/m2. The majority of participants were diagnosed with stage II (30%) or III (63%) breast cancer and received neoadjuvant chemotherapy (77%). High attendance to the 8-week HIIT intervention was 82.3% attained by the HIIT group31. The average range of RPE during HIIT was between 15–17 on the 6–20 scale among the participants. There were no adverse events reported over the duration of the intervention.
ECM-regulating enzyme results are displayed in Table 1. At baseline there were no differences in MMP (-1, -2, -7, -9, -10), TIMP-1 or TIMP-2 between the two groups. There was a significant within-group decrease in MMP-9 in the HIIT group [104.3 (51.9) to 65.2 (69.1); −37.4%; P = 0.01; d = 0.20) while MMP-9 in the CON group was not significantly changed [115.5 (47.2) to 90.4 (67.9); −21.7%; P = 0.10). Unexpectedly, a significant within-group increase in MMP-2 was observed in both HIIT group (8.6%; P = 0.007) and the CON group (12.6%; P = 0.003). Post-intervention, there was no significant within group or between group change in MMP-1, -7, and -10.
Discussion
Our results examined for the first time whether an 8-week HIIT exercise intervention influences ECM-regulating enzymes in cancer patients. While there was no significant between-group difference, an 8-week HIIT intervention significantly (within group differences) reduced MMP-9 levels in breast cancer patients undergoing anthracycline-based chemotherapy yet increased MMP-2 levels; no significant changes in MMP-1, -7, -10, TIMP-1 and TIMP-2 were observed. To our knowledge, this is the first study to examine exercise-related change in MMP-9 in cancer patients undergoing anthracycline-based chemotherapy.
The change in MMP-9 reported here is similar to previous exercise studies in non-cancer17 and cancer populations18 (the latter was an observational study). Kadoglou et al. (2010) reported a significant within-group decrease in serum MMP-9 after a 16-week supervised endurance training program (30–60 min of brisk walking; 4 days/week) in patients with type 2 diabetes17. In the observational study, Giganti et al. (2016) found that breast cancer patients who completed cancer treatment (10 years post-surgery) and who regularly participated in exercise (30 min treadmill plus 20 min strength training; 3 times a week) had significantly lower MMP-9 levels compared to breast cancer patients who did not participate in regular exercise18. However, this study lacked a baseline measure of MMP levels and did not involve a direct exercise intervention. Although it is difficult to compare our findings with the previous studies due to different exercise protocols, difference assessment of biomarkers and disease status (i.e. breast cancer vs type 2 diabetes), our study suggests that HIIT may partly contribute to the level of MMP-9 with a lower volume of exercise (90 min vs 150 min per week) over fewer number of weeks (8 vs 16 weeks). Further, we did not measure proBNP and cardiac troponinT; these serum biomarkers have been shown to predict cardiotoxicity in cancer patients32. It is noted that future clinical exercise trials may elucidate the effects of exercise on these cardiotoxicity markers in conjunction with the expression of MMP levels.
Potential biologic mechanisms may explain the exercise-induced changes in MMP-9. While we did not measure the level of inflammatory markers, a previous study reported that exercise training did not counteract the increase in inflammation, suggesting that beneficial effects of exercise on cardiotoxicity during breast cancer chemotherapy may not be regulated by inflammatory markers33. It is plausible that exercise may have reduced oxidative stress, thereby reducing MMP-9 levels34. Previous studies reported that MMP-9 was reduced in conjunction with tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) with no change in MMP-235,36. Evidence derived from a rat model indicated that MMP-9 expression is elevated by oxidative stress34,37,38, suggesting that the oxidative stress molecules38,39 may increase MMP-9 synthesis. It is possible that oxidative stress in the ECM were reduced by HIIT, which subsequently reduced MMP-9 levels. Future investigations are warranted to assess exercise-induced changes in oxidative stress with ECM-regulating enzymes to understand mechanism by which exercise influences MMP and TIMP levels.
While we hypothesized that HIIT would reduce MMP-2 levels in breast cancer patients undergoing anthracycline-based chemotherapy, MMP-2 levels were significantly increased in both the HIIT and CON groups. Although increases in MMP-2 levels are expected in patients undergoing anthracycline-based chemotherapy40,41, we expected the HIIT intervention would offset this increase. Thus, it appears that the exercise intervention was not adequate to offset the negative influence of anthracycline-based chemotherapy on this enzyme. This finding is consistent with a previous finding of Kadoglou et al.17 in that a 16-week supervised endurance training did not alter MMP-2 level in type 2 diabetics. In addition, Donley et al. (2014) reported that an 8-week aerobic exercise training (3 times/week, 60 min per day, 60–85% heart rate reserve) in cohorts with metabolic syndrome also did not change MMP-2 level7. Therefore, exercise, in general, may not be an effective non-pharmacologic strategy to target MMP-2 in breast cancer patients undergoing anthracycline-based chemotherapy. It is difficult, however, to compare these studies due to the variability across exercise protocols and clinical cohorts involved. Factors which still need to be considered before concluding that MMP-2 does not respond to exercise include the type, duration, frequency, and intensity of the exercise program, as well as the age and health status, of the cohort. Future studies are needed to examine how these multiple factors will influence MMP-2 levels in breast cancer patients who completed anthracycline-based chemotherapy.
Previous studies suggest that MMP-1, -7, -10 have a negative impact on cardiovascular disease such as hypertension42 or type 2 diabetes42,43,44. However, the present study did not find any significant changes in MMP-1, -7, -10 indicating that MMP-1, -7, -10 are not responsive to anthracycline-based chemotherapy and/or exercise training. A previous exercise intervention study in cohorts with metabolic syndrome conducted by Donley et al. reported findings consistent with our study showing that MMP-1, -7, -10 were not changed following an 8-week of aerobic exercise training7. Additional investigations are needed to elucidate the underlying exercise-induced effects on MMP-1, -7, -10.
Levels of TIMP-1 and -2 also did not change in either group implying that TIMP may not be influenced by anthracycline-based chemotherapy and/or HIIT. Our data suggest that the activity of TIMP is not influenced by anthracycline-based chemotherapy and/or HIIT. Our results are consistent with Donley et al. (2014) which determined that TIMP-1 and -2 were not significantly changed with exercise training in patients with metabolic syndrome7. Future studies are necessary to confirm whether TIMP-1 and -2 levels can be influenced by exercise and/or anthracycline-based chemotherapy.
The strengths of our study included: (1) direct one-on-one supervision of all exercise sessions, (2) high adherence of 82.3%; previous studies investigating exercised-related changes in MMP did not report the adherence to exercise7,17,18; (3) focus on a single chemotherapeutic agent so the effects of exercise on MMP with chemotherapy could be better understood.
Despite these strengths, we acknowledge the following limitations; (1) this study was designed as a pilot study, thus the small sample size is notable; (2) other related biomarkers were not investigated; (3) our patient population was mostly obese and may have been insulin resistant therefore, it is possible that our findings may be the indirect result of improvements in insulin sensitivity caused by exercise45.
In conclusion, it is unclear whether HIIT influences levels of MMP in breast cancer patients undergoing anthracycline-based chemotherapy yet MMP-2 was significantly increased both the HIIT and CON group; with no changes on MMP-1, -7, -10, TIMP-1 and -2. Further studies with an adequately powered sample size are required to identify an effective approach preventing side effects on ECM-regulating enzymes in breast cancer patients undergoing anthracycline–based chemotherapy.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sawyer, D. B. Anthracyclines and Heart Failure. N. Engl. J. Med. 368, 1154–6 (2013).
Plana, J. C. et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J-Card Img. 15, 1063–93 (2014).
Nikitovic, D. et al. Anthracycline-Dependent Cardiotoxicity and Extracellular Matrix Remodeling. Chest. 146, 1123–30. (2014).
Ishihara, H. et al. Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta(2) involves matrix metalloproteinases and tight junction proteins. J. Neuropath Exp. Neur. 67, 435–48 (2008).
Vacek, T. P. et al. Matrix metalloproteinases in atherosclerosis: role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc. health risk management. 11, 173–83 (2015).
Ezhov, M. et al. Matrix Metalloproteinase 9 as a Predictor of Coronary Atherosclerotic Plaque Instability in Stable Coronary Heart Disease Patients with Elevated Lipoprotein(a) Levels. Biomolecules. 9 (2019).
Donley, D. A. et al. Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J. Appl. physiology. 116, 1396–404 (2014).
Garvin, P., Nilsson, L., Carstensen, J., Jonasson, L. & Kristenson, M. Circulating matrix metalloproteinase-9 is associated with cardiovascular risk factors in a middle-aged normal population. PLoS one. 3, e1774 (2008).
Amin, M. et al. Regulation and involvement of matrix metalloproteinases in vascular diseases. Front. Biosci-Landmrk. 21, 89–118 (2016).
Vilela-Martin J., Cosenso-Martin, L. & Valente, F. Circulating lelels of matrix metalloproteinase-9 are elevated in individuals with hypertensive crisis. Journal of Hypertension. 36 (2018).
Olson, F. J. et al. Circulating matrix metalloproteinase 9 levels in relation to sampling methods, femoral and carotid atherosclerosis. J. Intern. medicine. 263, 626–35 (2008).
Kandasamy, A. D., Chow, A. K., Ali, M. A. M. & Schulz, R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc. Res. 85, 413–23 (2010).
Kelly, P. J. et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke - The biomarker evaluation for antioxidant therapies in stroke (BEAT-stroke) study. Stroke. 39, 100–4 (2008).
Nagareddy, P. R. et al. Inhibition of matrix metalloproteinase-2 improves endothelial function and prevents hypertension in insulin-resistant rats. Brit J. Pharmacol. 165, 705–15 (2012).
Baggen, V. J. M. et al. Matrix metalloproteinases as candidate biomarkers in adults with congenital heart disease. Biomarkers. 21, 466–73 (2016).
Peeters S. A. et al. Plasma matrix metalloproteinases are associated with incident cardiovascular disease and all-cause mortality in patients with type 1 diabetes: a 12-year follow-up study. Cardiovascular diabetology. 16 (2017).
Kadoglou, N. P. E. et al. Exercise ameliorates serum MMP-9 and TIMP-2 levels in patients with type 2 diabetes. Diabetes Metab. 36, 144–51 (2010).
Giganti, M. G. et al. Physical exercise modulates the level of serum MMP-2 and MMP-9 in patients with breast cancer. Oncol. Lett. 12, 2119–26. (2016).
Cockcroft, E. J. et al. High intensity interval exercise is an effective alternative to moderate intensity exercise for improving glucose tolerance and insulin sensitivity in adolescent boys. J. Sci. Med. Sport. 18, 720–4 (2015).
Adams, V. V. et al. High intensity interval training attenuates endothelial dysfunction in heart failure with preserved ejection fraction (HFpEF). Eur. J. Heart Fail. 17, 355 (2015).
Sawyer, B. J., Bhammar, D. M., Tucker, W. J. & Gaesser, G. A. Effects of High-Intensity Interval and Continuous Training on Endothelial Function and Glucose Regulation in Obesity. Med. Sci. sports exercise. 46, 863 (2014).
Currie, K. D., Bailey, K. J., Jung, M. E., McKelvie, R. S. & MacDonald, M. J. Effects of resistance training combined with moderate-intensity endurance or low-volume high-intensity interval exercise on cardiovascular risk factors in patients with coronary artery disease. J. Sci. Med. Sport. 18, 637–42 (2015).
Boyne, P. et al. High Intensity Interval Training May Be Superior to Moderate Intensity Continuous Exercise in Chronic Stroke. Stroke. 46 (2015).
Hallsworth, K. et al. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin. science. 129, 1097–105 (2015).
Lee, K. et al. Effects of high-intensity interval training on vascular function in breast cancer survivors undergoing anthracycline chemotherapy: design of a pilot study. BMJ open. 8, e022622 (2018).
Wright, D. B. Comparing groups in a before-after design: when t test and ANCOVA produce different results. Br. J. Educ. psychology. 76, 663–75 (2006).
Schneider, B. A., Avivi-Reich, M. & Mozuraitis, M. A cautionary note on the use of the Analysis of Covariance (ANCOVA) in classification designs with and without within-subject factors. Front. psychology. 6, 474 (2015).
Rietveld, T. & van Hout, R. The t test and beyond: Recommendations for testing the central tendencies of two independent samples in research on speech, language and hearing pathology. J. Commun. disorders. 58, 158–68 (2015).
Larner, A. J. Effect Size (Cohen’s d) of Cognitive Screening Instruments Examined in Pragmatic Diagnostic Accuracy Studies. Dement. geriatric Cognit. Disord. extra. 4, 236–41 (2014).
Lee, K. et al. Effects of high-intensity interval training on vascular endothelial function and vascular wall thickness in breast cancer patients receiving anthracycline-based chemotherapy: a randomized pilot study. Breast cancer research and treatment. (2019).
Lee, K. et al. Feasibility of high intensity interval training in patients with breast Cancer undergoing anthracycline chemotherapy: a randomized pilot trial. BMC cancer. 19, 653 (2019).
Garrone, O. et al. Prediction of anthracycline cardiotoxicity after chemotherapy by biomarkers kinetic analysis. Cardiovascular toxicology. 12, 135–42 (2012).
van Vulpen, J. K. et al. Effects of physical exercise on markers of inflammation in breast cancer patients during adjuvant chemotherapy. Breast cancer Res. treatment. 168, 421–31. (2018).
Oh, C., Dong, Y. & Thompson, L. Chronic hypoxia increases IL-6, TNF-, and matrix metalloproteinases (MMP2 and MMP9) expression of fetal guinea pig hearts. Am. J. Obstet. Gynecol. 195, S9–S (2006).
Ohta, K. et al. TNF–induced IL-6 and MMP-9 expression in immortalized ameloblastoma cell line established by hTERT. Oral. Dis. 23, 199–209 (2017).
Ma, C. G. et al. The suppression of MMP-9 and proinflammatory cytokines IL-1 beta, TNF-alpha and IL-6 contributes to the protective effect of triptolide in primary astrocytes after hypoxia/reoxygenation injury. J. Neurol. Sci. 285, S310–S1 (2009).
Manicone, A. M. & McGuire, J. K. Matrix metalloproteinases as modulators of inflammation. Semin. Cell Dev. Biol. 19, 34–41 (2008).
Pustovrh, M. C. et al. Oxidative stress promotes the increase of matrix metalloproteinases-2 and -9 activities in the feto-placental unit of diabetic rats. Free. Radic. research. 39, 1285–93 (2005).
Reihmane, D., Jurka, A., Tretjakovs, P. & Dela, F. Increase in IL-6, TNF-alpha, and MMP-9, but not sICAM-1, concentrations depends on exercise duration. Eur. J. Appl. physiology. 113, 851–8 (2013).
Ivanova, M. et al. Chronic cardiotoxicity of doxorubicin involves activation of myocardial and circulating matrix metalloproteinases in rats. Acta Pharmacol. Sin. 33, 459–69 (2012).
Potacova, A. et al. Cardiac remodeling and the role of matrix metalloproteinases in chronic anthracycline cardiotoxicity. J. Mol. Cell Cardiol. 40, 1001 (2006).
Berry, E., Bosonea, A. M., Wang, X. & Fernandez-Patron, C. Insights into the Activity, Differential Expression, Mutual Regulation, and Functions of Matrix Metalloproteinases and A Disintegrin and Metalloproteinases in Hypertension and Cardiac Disease. J. Vasc. Res. 50, 52–68 (2013).
Soliman, A. R., Sadek, K. M., Thabet, K. K., Ahmed, D. H. & Mohamed, O. M. The Role of Matrix Metalloproteinases 2 in Atherosclerosis of Patients with Chronic Kidney Disease in type 2 diabetes. Saudi J. Kidney Dis. T. 30, 387–93 (2019).
Peeters, S. A. et al. Plasma matrix metalloproteinases are associated with incident cardiovascular disease and all-cause mortality in patients with type 1 diabetes: a 12-year follow-up study. Diabetologia. 60, S512–S3 (2017).
Unal, R. et al. Matrix Metalloproteinase-9: Association with Insulin Resistance and Obesity and PKC alpha Mediated Regulation by Pioglitazone. Diabetes. 58, A355–A6 (2009).
Acknowledgements
We acknowledge the Clinical Investigations Support Office of the Norris Comprehensive Cancer Center for their regulatory support of this investigation and the extraordinary generosity of our study participants. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number P2CHD086851 and by grant UL1TR001855 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Conceptualization (All authors); Methodology (K.L., I.K., G.S., W.M., F.S. and C.D.C.); Writing Original Draft (all authors); Writing Review & Editing (All authors); Resources (C.D.C.); Supervision (J.M., C.D.C.). All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, K., Kang, I., Mack, W.J. et al. Effect of High Intensity Interval Training on Matrix Metalloproteinases in Women with Breast Cancer Receiving Anthracycline-Based Chemotherapy. Sci Rep 10, 5839 (2020). https://doi.org/10.1038/s41598-020-61927-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61927-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.