Abstract
Background
The increase in fibronectin type-III domain-containing protein 5 (FNDC5), myonectin, and glucose transporter 4 (GLUT4) leads to a decrease in diabetes; meanwhile, exercise training can affect these factors. The result regarding the comparison between the effect of high-intensity interval training (HIIT) and that of moderate-intensity continuous training (MICT) is confusing. Thus, the present study investigated the comparative effects of HIIT and MICT on soleus muscle FNDC5, myonectin, and GLUT4 gene expressions in the diabetic rat model.
Methods and results
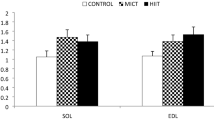
Seventy-two male Wistar rats (weight 200 g ± 20) were randomly and equally assigned to six groups: control-healthy, MICT-healthy, HIIT-healthy, control-diabetes, MICT-diabetes, and HIIT-diabetes. At the first level, Streptozotocin (STZ) was utilized to induce diabetes in rats (at a dose of 55 mg/kg). After that, the training groups performed HIIT and MICT programs on the rodent treadmill for 6 weeks (five-session/week). Twenty-four hours after the last intervention, soleus muscle was removed, and sent to a research facility for future examinations. HIIT and MICT increased the muscle FNDC5, myonectin, and GLUT4 gene expression compared to the control group (P < 0.05). The type of training had no significant effect on the FNDC5 (P > 0.05), while the MICT program induced a greater increase in the myonec ztin and GLUT4 compared to the HIIT program (P < 0.05). Meanwhile, a positive relationship between all variables was observed.
Conclusions
Exercise training has a beneficial effect on diabetes conditions via the effect of FNDC5, myonectin, and GLUT4. Due to the correlation between myonectin and GLUT4 shown in the present study, physical activity may alter myonectin through its effect on GLUT requiring further investigation by subsequent studies.

Similar content being viewed by others
Data availability
All raw data used to analyze these results are freely available on reasonable request.
References
Glovaci D, Fan W, Wong ND (2019) Epidemiology of diabetes mellitus and cardiovascular disease. Curr Cardiol Rep 21(4):1–8
Shoaib A et al (2020) Increased serum lipid profile and development of vascular complications in diabetic individuals—a comparative study. PJMHS 14(4):1–2
Dimitriadis GD et al (2021) Regulation of postabsorptive and postprandial glucose metabolism by insulin-dependent and insulin-independent mechanisms: an integrative approach. Nutrients 13(1):159
Stanford KI, Goodyear LJ (2014) Exercise and type 2 diabetes: molecular mechanisms regulating glucose uptake in skeletal muscle. Adv Physiol Educ 38(4):308–314
Rodríguez A et al (2017) Crosstalk between adipokines and myokines in fat browning. Acta Physiol 219(2):362–381
Gamas L, Matafome P, Seiça R (2015) Irisin and myonectin regulation in the insulin resistant muscle: implications to adipose tissue: muscle crosstalk. J Diabetes Res 2015:359159–359159
Arhire LI, Mihalache L, Covasa M (2019) Irisin: a hope in understanding and managing obesity and metabolic syndrome. Front Endocrinol. https://doi.org/10.3389/fendo.2019.00524
Chen N et al (2016) Irisin, an exercise-induced myokine as a metabolic regulator: an updated narrative review. Diabetes Metab Res Rev 32(1):51–59
Perakakis N et al (2017) Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol 13(6):324–337
Gizaw M, Anandakumar P, Debela T (2017) A review on the role of irisin in insulin resistance and type 2 diabetes mellitus. J Pharmacopuncture 20(4):235–242
Xin C et al (2016) Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes (Lond) 40(3):443–451
Seldin MM et al (2012) Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 287(15):11968–11980
Gamas L, Matafome P, Seiça R (2015) Irisin and myonectin regulation in the insulin resistant muscle: implications to adipose tissue: muscle crosstalk. J Diabetes Res. https://doi.org/10.1155/2015/359159
Tayebi SM et al (2019) Plasma retinol-binding protein-4 and tumor necrosis factor-α are reduced in postmenopausal women after combination of different intensities of circuit resistance training and Zataria supplementation. Sport Sci Health 15(3):551–558
Brandt C, Pedersen BK (2010) The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol. https://doi.org/10.1155/2010/520258
Mathur N, Pedersen BK (2008) Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm. https://doi.org/10.1155/2008/109502
Arabzadeh E et al (2020) Alteration of follistatin-like 1, neuron-derived neurotrophic factor, and vascular endothelial growth factor in diabetic cardiac muscle after moderate-intensity aerobic exercise with insulin. Sport Sci Health. https://doi.org/10.1007/s11332-020-00631-9
Fisher G et al (2015) High Intensity interval- vs moderate intensity-training for improving cardiometabolic health in overweight or obese males: a randomized controlled trial. PLoS One. https://doi.org/10.1371/journal.pone.0138853
Stein RA et al (1990) Effects of different exercise training intensities on lipoprotein cholesterol fractions in healthy middle-aged men. Am Heart J 119(2 Pt 1):277–283
O’Donovan G et al (2005) Changes in cardiorespiratory fitness and coronary heart disease risk factors following 24 wk of moderate- or high-intensity exercise of equal energy cost. J Appl Physiol. https://doi.org/10.1152/japplphysiol.01310.2004
Rahmati-Ahmadabad S et al (2019) High-intensity interval training has a greater effect on reverse cholesterol transport elements compared with moderate-intensity continuous training in obese male rats. Eur J Prev Cardiol. https://doi.org/10.1177/2047487319887828
Tine Kartinah N et al (2018) The effects of exercise regimens on irisin levels in obese rats model: comparing high-intensity intermittent with continuous moderate-intensity training. Biomed Res Int 2018:4708287
Teimourian M, Fatolahi H, Mateenhomaei H (2020) Effect of different exercise mode and ursolic acid supplementation on FNDC5 and UCP1 gene expression and plasma irisin in rats. Int J Sports Exerc Med. https://doi.org/10.23937/2469-5718/1510160
Chavanelle V et al (2017) Effects of high-intensity interval training and moderate-intensity continuous training on glycaemic control and skeletal muscle mitochondrial function in db/db mice. Sci Rep 7(1):204
Arabzadeh E, Mirdar S, Fathi Z (2015) Measurement of levels of lung HIF-1α protein in response to tapering for 14-and 21-day with nigella sativa supplementation in maturing rat, with histological study. Sport Sciences for Health 11(2):195–202
Arabzadeh E, Mirdar S, Moradiani H (2016) Nigella sativa supplementation attenuates exercise-induced bronchoconstriction in the maturing rat: a histometric and histologic study. Comp Clin Pathol 25(1):1–5
Yoon H et al (2015) Moderate exercise training attenuates inflammatory mediators in DRG of Type 1 diabetic rats. Exp Neurol 267:107–114
Grant CW et al (2012) Development of standardized insulin treatment protocols for spontaneous rodent models of type 1 diabetes. Comp Med 62(5):381–390
Bedford TG et al (1979) Maximum oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol 47(6):1278–1283
Leandro CG et al (2007) A program of moderate physical training for Wistar rats based on maximal oxygen consumption. J Strength Cond Res 21(3):751
Lade CG et al (2018) Effects of moderate intensity endurance training vs high intensity interval training on weight gain, cardiorespiratory capacity, and metabolic profile in postnatal overfed rats. Diabetol Metab Syndrome. https://doi.org/10.1186/s13098-018-0374-x
Kurdiova T et al (2014) Exercise-mimicking treatment fails to increase Fndc5 mRNA & irisin secretion in primary human myotubes. Peptides 56:1–7
Timmons JA et al. (2012) Is irisin a human exercise gene? Nature 488(7413):E9–10; discussion E10–1
Park KH et al (2013) Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab 98(12):4899–4907
Li K et al (2017) Myonectin predicts the development of type 2 diabetes. J Clin Endocrinol Metab 103(1):139–147
Bostrom P et al (2012) A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481(7382):463–468
Erickson HP (2013) Irisin and FNDC5 in retrospect: an exercise hormone or a transmembrane receptor? Adipocyte 2(4):289–293
Gouni-Berthold I et al (2013) Effects of lipid-lowering drugs on irisin in human subjects in vivo and in human skeletal muscle cells ex vivo. PLoS ONE. https://doi.org/10.1371/journal.pone.0072858
Huh JY et al (2014) Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab 99(11):E2154–E2161
Lee HJ et al (2015) Irisin, a novel myokine, regulates glucose uptake in skeletal muscle cells via AMPK. Mol Endocrinol 29(6):873–881
Yang Z et al (2015) Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int J Clin Exp Pathol 8(6):6490–6497
Funding
No funding.
Author information
Authors and Affiliations
Contributions
HSH conceived the study. SRA and FR carried out the experiments. GHM and SRA provided methodological advice and materials. HSH and FR wrote the manuscript. All authors agreed upon the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted following the National Institutes of Health (NIH) guidelines. Moreover, this study was performed with the written permission of the research deputy of Baqiyatallah University and has an ethical code (IR.BMSU.REC.1396.649).
Consent to participate
Animal study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahmati-Ahmadabad, S., Rostamkhani, F., Meftahi, G.H. et al. Comparative effects of high-intensity interval training and moderate-intensity continuous training on soleus muscle fibronectin type III domain-containing protein 5, myonectin and glucose transporter type 4 gene expressions: a study on the diabetic rat model. Mol Biol Rep 48, 6123–6129 (2021). https://doi.org/10.1007/s11033-021-06633-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06633-1