Abstract
The electrodeposition of aluminum on mild steel in a molten salt electrolyte consisting of a mixture of AlCl3/NaCl/KCl (weight percent ratio of 80:10:10) was studied. Parametric studies were carried out to evaluate the effect of different parameters such as current density, electrolysis time, and intermediate coating layer on the coating morphology and coating-to-substrate adhesion. The quality and morphology of the coating were investigated using scanning electron microscope (SEM) and x-ray map analyses. The effect of heat treatment of the coated samples on the interface stability and formation of intermetallic compounds at the Al-Fe interface was also investigated. Cross-sectional examination by SEM as well as energy dispersive spectroscopy (EDS) line scan showed that upon annealing at temperatures in the range of 350 °C–550 °C, brittle Fe-Al intermetallic layers were formed at the interface. This shows that high-temperature service conditions can adversely affect the coating properties. The apparent activation energy of the formation of such intermetallic layers was calculated based on thickness measurements on these layers. The optimum conditions for electroplating were determined as current density of 0.022 A.cm−2 and electroplating time of 60 min. Potentiodynamic polarization tests were used to evaluate the corrosion resistance of the samples in 3.5 wt% NaCl solution. Considering the corrosion rate of coated samples which is much lower than the bare substrate, it was concluded that the electrodeposited coatings could efficiently protect the steel substrate against corrosion in corrosive media.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Aluminum is considered as one of the most important metals in the industry due to its excellent combination of properties such as low density, high ductility, and high electrical and thermal conductivity [1–3]. Its outstanding resistance to corrosion makes it one of the most widely-used metals in protective coatings [4]. There are several industrial methods for developing aluminum-containing coatings on a metal surface such as hot dipping [5], chemical and physical vapour deposition (CVD/PVD) [6, 7], and thermal spraying [8]. Most of these methods need to be carried out at high temperatures because of relatively high melting point of aluminum (660 °C); they are also not suitable for applying coatings on complex geometries [4]. Many of the coating methods suffer problems such as difficulties in controlling the coating thickness and loss of material and energy. In comparison, electroplating presents high efficiencies and low operating costs; besides, lower levels of residual stress are built up in the substrate because of low processing temperatures [9]. Therefore, deposition of aluminum by electrolysis has been known as one of the most practical methods for applying aluminum coatings on different substrates. The electrodeposition of aluminum from aqueous solutions is not possible due to its high reactivity which gives rise to the reduction and evolution of hydrogen gas instead of reduction of aluminum on the cathode [10, 11]. Instead, non-aqueous solvents such as ionic liquids [12] (which are classified into three categories, namely, Alkylpyridinium aluminates such as ethylpyridinium bromide and N-(1-butyl) pyridinium chloride [13–15], Alkyl imidazolium aluminates such as 1-ethyl-3-methyl imidazolium chloride [16, 17], and trimethyl phenylammonium chloride [18, 19]) or organic solvents (also categorized as etheric solvents, dimethylsulfone, and aromatic hydrocarbons) [20–23] or molten salts have to be used as electrolyte.
Common molten salts used for aluminum electrodeposition are AlX3.MX melts where X can be F− [24], B− [25] or Cl−, and M is an alkali ion (N+, K + or Li+) [4]. The electrodeposition of Al-Ta [26], Al-Mn [27], Al-Cu [28], Al-Sn [29], Al-Cr [30], Al-Cu [31], Al-Mn-Zr [32], Mg-Li-Al [33], and Al-Pb [34] alloys has been possible using this method. Molten salt electrodeposition (MSE) method offers advantages such as: high ionic conductivities (2–9 Ω−1cm−1) and diffusivities with low viscosities of molten alkali and alkaline metal salts and their mixtures; moreover, overpotential values resulting from cathodic polarization are often small in cathodic deposition, which is desirable from an energy viewpoint [35].
Despite numerous published studies on the electrodeposition of aluminum on steel, the effect of process parameters and heat-treatment temperature on the electrodeposited Al coatings on mild steel from the specific salt composition used in this study have not been investigated. In this paper, the electrodeposition of aluminum on mild steel substrates in an AlCl3–KCl–NaCl molten salt electrolyte was carried out. The effect of electrolysis time, current density, and copper and zinc interlayers on the morphology and adhesion properties of the coating was studied at constant bath constituents. The influence of heat treatment on the formation of undesired intermetallic phases between Al and Fe at the interface was evaluated. The corrosion behaviour of coated samples was also compared with the uncoated specimens.
2. Experimental
The substrate used in this study was mild steel (C 0.24wt%, Si 0.18 wt%, Mn 0.73wt%, S 0.02 wt%). The specimens were mechanically polished to 1200-grit SiC paper and then immersed in 20 wt% NaOH solution for 10 min to eliminate any grease on their surface. They were then immersed in 30 wt% HCl to activate their surface. Finally, the specimens were placed in an ultrasonic bath. Commercially pure aluminum with a minimum of 99.0% Al was used as anode. To prepare the electrolyte, 80 wt% AlCl3 with 10 wt% NaCl and 10 wt% KCl (>99.5% purity, MERCK) were mixed together. To improve coating quality and obtain more compact coatings during the electrodeposition process, it is common to use appropriate additives to the electrolyte. The choice of additives mainly depends on electrolytes because of the discrepancies in their solubilities. A suitable group of additives are alkali halides such as KI and NaI, which have proved to be very effective due to their acceptable solubility and low volatility. In presence of additive, the number of charged particles in electrolyte would be increased; therefore, the conductivity of the electrolyte rises. The positive effect of such additives has been mainly attributed to two mechanisms [36, 37]: (a) they prevent nucleation and limit growth by absorption onto the surface, (b) they form complex metal ions and increase their reduction potential, therefore, make it more difficult for metals clusters to nucleate. The I− ion forms AlI− 4 complex anions; it could also be absorbed on the surface and prevent cathodic reduction reaction, promote nucleation by homogenization of cathode surface energy, and inhibit spongy deposition. It prevents nuclei growth by blocking the active sites on cathode surface and increases the number of growing nuclei [36–39]. In this study, 5% wt. KI was added to the electrolyte. An electrolyte with such composition is melted at approximately 120 °C. However, due to the higher vapour pressure of Al2Cl6 at lower temperatures, the process temperature was set at 175 °C. The electrolysis cell was a Pyrex Becher that was placed in an oil bath to ensure constant temperature during electrolysis. The oil bath was in turn placed on a heater. The electrodeposition process was carried out at the specified temperature and time. The effect of current density (0.003, 0.022, and 0.1 A.cm−2), deposition time (15, 30, and 60 min), and intermediate layer (Zn, Cu) on coating morphology and particle size was studied.
After electrodeposition, the cathode was removed from electrolyte and washed with ethanol (C2H5OH). In order to study the high-temperature phenomena at Al/steel interface, the specimens were heat treated for 30 min in a tube furnace with argon atmosphere. The heat treatment was carried out at three temperatures, namely, 350 °C, 450 °C, and 550 °C. Examination of surface and cross section morphologies, EDS line scan, and elemental x-ray mapping were carried out by scanning electron microscope (SEM: TESCAN VEGA//XMU, TESCAN VEGA//LMU).
Potentiodynamic polarization tests in 3.5 wt% NaCl solution were performed at room temperature using a Princeton Applied Science Potentiostat to study the corrosion behaviour of the specimens.
To study the effect of zinc interlayer, zinc sulphate solution was used for the electroplating of a thin zinc layer at 0.28 A.cm−2 current density for 5 min before the electrodeposition of aluminum on the electrode. The prepared sample was electrolyzed under the mentioned conditions and the zinc coating was formed on the substrate. Copper cyanide solution (27 g L−1 CuCN, 54 g L−1 NaCN, 16 g L−1 NaOH) at 0.16 A.cm−2 current density and 5 min duration was used for the electroplating of a copper interlayer.
3. Results and discussion
Aluminum electrodeposition was carried out under the determined conditions, and the effect of various parameters on the deposition process was evaluated using a one-parameter-at-a-time approach.
3.1. Current density
Current density has a determinative effect on the coating brightness, deposition rate, thickness uniformity, current efficiency, particle size, and coating microstructure. In this study, current density values of 0.003 A.cm−2, 0.022 A.cm−2, and 0.1 A.cm−2 were applied to investigate the effect of variations in current density on the current efficiency, coating morphology, and particle size.
The current efficiency in the aluminum electrodeposition process is defined as the yield of aluminum coating on the cathode under different experimental conditions, and is mainly affected by the cathodic reactions and nucleation and growth of aluminum particles [9]. The current efficiency can be calculated using Faraday's laws in electrochemistry. As the current density increases, the total charge passing through the cell increases, and the theoretical aluminum deposition would be increased too; however, the actual mass of deposited aluminum is much slower than its theoretical mass due to the current loss (polarization effect) during the electrodeposition process. To calculate the mass of deposited aluminum, the reduction in weight of coated electrodes after stripping of the specimens in 0.5 wt% sodium hydroxide solution was measured. It was observed that by increasing the current density from 0.022 (A.cm−2) to 0.032 (A.cm−2), the current efficiency would decrease from 90% to 80%. It can be seen that there is an optimum value for the current density in the deposition of aluminum from mixture of molten salts. This may be elaborated by studying the effect of current density on the nucleation and growth process during the deposition of aluminum. At very low values of current density, discharging the ions on the cathode occurs slowly so that the rate of nuclei growth would be larger than the rate of formation of new nuclei [40], and the deposited particles would be larger than their sizes at higher current density. Also with increasing current density, the cathode overvoltage as well as the driving force for nucleation increases [41]; therefore, the nucleation rate will become higher leading to the increase in the number of nuclei, resulting in the deposition of aluminum as finer particles. The coating particle size is compared in SEM micrographs presented in figure 1 for two different values of applied current density, from which the differences in particle size are evident. Image J image analysis software was used for the calculation of average surface area which is related to the size of particles, and average surface areas of 1.027 μm2 and 0.573 μm2 were obtained for the current densities of 0.022 A.cm−2 and 0.032 A.cm−2, respectively.
Figure 1. SEM micrographs showing the deposited aluminum particles at different current densities, (a) 0.022 A.cm−2 (b) 0.032 A.cm−2.
Download figure:
Standard image High-resolution imageThe overall morphology of the coating is also influenced by current density. Variation of current density is limited because of the dendritic growth threshold current density (DGTCD), the amount of DGTCD depending on various factors like substrate material [42], rotation speed of electrode [43], and electrolyte composition [44, 45]. It has been found out that upon approaching the value of current density to DGTCD, aluminum coating is deposited in a spongy or dendritic form. The shapes of the dendrites are mainly determined by the directions of preferred growth in the lattice [46]. At too low current densities, the number of critical nuclei which operate as growth centre is small, resulting in a spongy coating. The reason for dendritic structure of deposition is a slower rate of mass transfer and electrical charge transfer than the deposition rate of metal atoms. In order to reach a smooth and fine structure and a high-quality coating, it is favourable to apply current densities in the range of 0.02–0.05 A.cm−2. Below the 0.02 A.cm−2 limit, spongy deposits and above 0.08 A.cm−2, dendritic deposits are obtained [21, 36, 46]. At low values of current density, the deposition of aluminum is not fulfilled in all surface sites because of low cathode overpotentials, whereas at high current densities larger cathode overpotential causes the variation of initial aluminum deposition, hence the deposits grow uniformly in different directions. However, too high cathode overpotentials result in non-uniform distribution of current density, high deposition rate, and dendritic growth.
It can be observed in figure 2 that at current densities as low as 0.003 A.cm−2, spongy deposition and at high densities such as 0.1 A.cm−2 coarse, dark grey dendrites were obtained.
Figure 2. Aluminum coating morphology at different current densities, (a): 0.003 A.cm−2, (b) 0.022 A.cm−2, (c) 0.1 A.cm−2.
Download figure:
Standard image High-resolution imageThe micrographs in figure 3 also compare higher-magnification micrographs of the coating obtained at high and low values of current density, in which the dendritic growth at high current density values is evident.
Figure 3. Electrodeposited layer in various current densities, (a), (c) 0.1 A.cm−2 (b), (d) 0.022 A.cm−2.
Download figure:
Standard image High-resolution image3.2. Electrodeposition time
It is obvious the the coating thickness is affected by the time of electrolysis. At low electrodeposition times, the growth of the nuclei, occupation of sites, and coverage of the surface would not be accomplished. Different electrolysis times of 15, 30, and 60 min were examined in this study at a constant current density of 0.022 A.cm−2. It has been found out the formation and growth of deposited aluminum follows a 3D-island model. This formation occurs through four stages: (1) formation of isolated nuclei and their growth in the form of three-dimensional crystallites (TDC), (2) coalescence of TDCs, (3) formation of a linked network, and (4) formation of the continuous deposit [47]. During the formation of islands, the free energy of the system changes [48]. The initial stages have rarely been observed due to rapid coating formation and high density of formed nuclei.
Each island that forms on the substrate undergoes growth in both vertical and horizontal directions. As shown in figure 4, the structure of coating during the growth would be in the form of numerous semi-conical columns with their tops up (figure 4(a)). Over time, these cones tend to coalesce and a continuous film is formed due to charge density; however, the particle growth seems to follow the Volmer-Weber growth mode, in which the atoms are more strongly coupled with each other than with the substrate [49]. Ultimately after 60 min, coherent, smooth, and homogeneous deposits are obtained.
Figure 4. SEM micrographs of aluminum coating in various electroplating times, (a) 15 min, (b) 30 min (c) 60 min.
Download figure:
Standard image High-resolution imageIn the island 3 growth mode, increased coating thickness and complete covering of the surface will be accomplished by the growing islands. In figure 4 and figure 5, island growth by the passage of time from 15 min to 30 min is evident. These micrographs show that growth is a function of time, which is consistent with the Avrami analysis for island growth [50].
Figure 5. Cross sectional morphology of aluminum coating in various times, (a) 15 min, (b) 30 min, (c) 60 min.
Download figure:
Standard image High-resolution imageAnother effect of time on the coating characteristics is in terms of particle size. As schematically shown in figure 6, by passage of time, islands will grow and overlap leading to an increase in their density. Particle size or in other word, inter-island spacing (λ) is adversely related to islands density (λ = N−1/2) [50]. In figure 4, the reduction in particle sizes with passage of time is clear.
Figure 6. Schematic of growth and overlapping islands with passage of the time.
Download figure:
Standard image High-resolution image3.3. Intermediate layers
It has been reported in the literature that the existence of an intermediate layer between the substrate and the coating can positively influence the quality of coatings [51], offer better corrosion resistance [52], and/or prevent dissolution of the substrate surface during the coating process [27]. The adhesion of aluminum coating to the substrate which was evaluated by studying the cross section of specimens (figure 5(c)) showed that there is already a good bonding and adhesion between the substrate and coating, and no cracks and brittle intermetallic phases are observed at the interface. Nonetheless, the effect of copper and zinc as common intermediate layers in electrodeposition process on the coating quality was investigated.
The morphology of aluminum coatings in the presence of copper and zinc interlayers can be seen in the micrographs of figure 7, The growth of aluminum on the copper layer (figures 7(a) and (b)) seems similar to its growth on steel (figure 4(c)) and the presence of copper has no significant effect on the coating morphology, whereas on the zinc layer hemispherical island formation occurred leading to Volmer-Weber growth mode and a non-uniform aluminum deposit which were also reported by Y Hang [53]. This shows that the nature and surface structure of substrate can affect the coating morphology.
Figure 7. SEM morphology of coating, (a), (b) copper intermediate layer, (c), (d) zinc intermediate layer.
Download figure:
Standard image High-resolution imageBy studying the Al/Zn cross section and the x-ray elemental maps of the cross section (figure 8), it was found out that part of Zn was dissolved in the Al coating, forming an Al-Zn alloy.
Figure 8. SEM micrograph of the cross section of steel electrode coated with Zn and Al (a); x-ray elemental maps showing the distribution of Al (b), Fe (c), and Zn (d).
Download figure:
Standard image High-resolution imageThe interface between zinc and aluminum layers shows deficient bonding, and nucleation of aluminum at early stages was incomplete because of the low activity of the zinc layer for aluminum nucleation.
Copper intermediate layer seemed to have a higher activity for aluminum nucleation than zinc, hence more effective coating process was possible in the presence of a copper intermediate layer. However, the thickness of the copper layer decreases upon the placement of the electrode in the electrolyte as shown in figure 9, which may be indicative of the dissolution of copper in the molten metal bath during the electrodeposition process. In constant conditions, aluminum coating with a lower thickness was obtained in the presence of a copper intermediate layer. Figure 9 shows that the approximate thickness of the aluminum coating is 0.8 μm in the presence of interlayer, whereas without any intermediate layer the thickness of the obtained aluminum layer was ca. 24.8 μm.
Figure 9. Cross section of the specimen with copper intermediate layer and aluminum coating.
Download figure:
Standard image High-resolution imageBased on the observations in this study, the application of intermediate layers did not have any positive effect on the adhesion of coating.
3.4. Heat treatment
According to the Fe-Al binary phase diagram, several intermetallic compounds such as Fe3Al, FeAl, Fe2Al5, and FeAl3 can be formed as a result of interactions between Fe and Al [54]. FeAl3 mostly forms near the aluminum side, Fe2Al5 at the centre, and FeAl and Fe3Al near the iron side. FeAl3 and Fe2Al5 are hard and brittle, whereas Fe3Al and FeAl are ductile. Therefore, the intermetallic layer can be divided into two ductile and brittle zones [55]. It should be noted that the formation of an intermetallic layer depends on three factors: chemical potential of aluminum and iron elements, nucleation of phases at the beginning of the interdiffusion process, and mobility of the intermetallic layer elements during heat treatment [56]. Herein aluminum-coated steel specimens were annealed at three temperatures: 350°, 450°, and 550 °C for 30 min in argon atmosphere to study the formation of Fe-Al intermetallics at the interface. Chemical composition of the formed layers was determined using EDS analysis. The results of EDS line scan showing the concentration profile of the elements across the interface are shown in figure 10. It was found out that the intermetallic layer formed at the Fe/Al interface is composed of two phases, the chemical compositions of which correspond to FeAl3 and Fe2Al5. Due to the small thickness of Fe2Al5 and especially FeAl3 layers, the total thickness of Fe2Al5 and FeAl3 is reported as the intermetallic layer thickness. The enthalpies of formation of FeAl3 and Fe2Al5 are −112.56 and −194.04 kJ.mol−1, respectively [57], which implies that the formation of FeAl3 is thermodynamically more favourable and its formation can occur at the early stages of heating, while the larger fraction of Fe2Al5 in the intermetallic layer is because of its higher kinetic growth rate. As shown in figure 10 with an increase in annealing temperature, the thickness of intermetallic layer increases because of facilitated diffusion of elements.
Figure 10. SEM micrograph showing the cross section and EDS line analysis after heat treatment, (a) 350° C, (b) 450° C, (c) 550° C.
Download figure:
Standard image High-resolution imageSolid state reactions, which are mostly controlled by diffusion, play a significant role in the rate of reaction between two reacting solids [58]. Growth rate of the intermetallic layer is dependent on the rate of diffusion and rate of chemical reaction [59]. Since Fe2Al5 is the major phase in the intermetallic layer, the overall growth rate of intermetallic layer can be approximated by the growth rate of Fe2Al5 layer. The compliance of growth rate of Fe2Al5 layer to the parabolic law at solid state is supported by the data in the literature [60–62]. The thickness of Fe2Al5 layer is directly proportional to the square root of time:
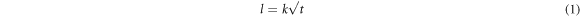
where l is the intermetallic thickness, t is the annealing time and k is the growth rate constant. The value of k depends on the temperature according to the Arrhenius equation [60]:

where k0 is pre-exponential factor, Q is the activation energy (kJ mol−1), T is the absolute temperature (K) and R is the universal gas constant.
To calculate the thickness of intermetallic layer, the thickness of intermetallic layer at twenty points was measured and averaged. The growth constant (μm.s−1/2) was derived from the parabolic law for samples at three temperatures, and values of 0.042 for 350 °C, 0.068 for 450 °C and 0.253 for 550 °C were obtained for times up to 30 min.
The apparent activation energy for the interfacial formation of Fe-Al intermetallic compounds can be obtained from the slope of ln (k)—T−1 plot as shown in figure 11. Based on the data obtained after heat treatment at 350 to 550 °C, the activation energy was calculated as 74.2 kJ mol−1. This relatively low activation energy value is expectable because of rapid sideway growth of intermetallic layer.
Figure 11. Calculation of the activation energy for the formation of Fe-Al intermetallics at the Fe/Al interface.
Download figure:
Standard image High-resolution image3.5. Corrosion resistance properties
In order to evaluate the performance of the coated electrodes in service conditions, potentiodynamic polarization curves of typical samples in a 3.5 wt% NaCl solution at room temperature were compared (figure 12). Four samples were chosen to perform the corrosion tests: sample (1): Al coating, 0.022(A.cm−2), 60 min; sample (2): Al coating, 0.003(A.cm−2), 60 min; sample (3): bare steel substrate; sample (4): annealed aluminum coating, 0.022(A.cm−2) at 350 °C for 30 min.
Figure 12. Potentiodynamic polarization curves of various specimens in 3.5% NaCl solution.
Download figure:
Standard image High-resolution imageThe corrosion potential (Ecorr), corrosion current density (icorr), and anodic/cathodic Tafel slopes ( and
and  ) were derived from the data. The polarization resistance (Rp) values were determined from Stern–Geary equation (equation (3)) [63]:
) were derived from the data. The polarization resistance (Rp) values were determined from Stern–Geary equation (equation (3)) [63]:
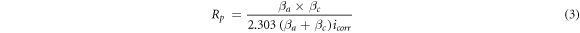
The results of potentiodynamic polarization tests are shown in table 1. Figure 12 and table 1 indicate that the corrosion rate (icorr) of steel substrate was higher than the coating, and also the value of Rp for sample (1) with optimum conditions of coating is the highest among the studied samples. It can be seen that the corrosion resistance of the substrate was improved by applying an aluminum coating on it. Comparison of samples (1) and (2) indicates that the values of 0.022 (A.cm−2) for the current density and 60 min of coating duration which present the best coating quality and uniform morphology, also yield the highest corrosion resistance. Sample (2) has a spongy and non-uniform coating with low thickness, hence lower corrosion resistance. Sample (4) has higher corrosion rate than samples (1) and (2), because of the formation of brittle intermediate layers at the interface of the coating and the substrate that eventually leads to the weakening of coating adhesion. As shown in figure 12, there is a small peak in the curve of the sample (4) that refers to the intermediate layer decomposition during the corrosion process. Therefore, the annealed specimens do not show acceptable resistance to corrosion.
Table 1. Results of potentiodynamic corrosion tests of different specimens in 3.5% NaCl.
| Specimen | Ecorr (V) | icorr (A/cm−2) |
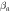 (V) (V) | Rp (Ω.cm2) |
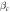 (V) (V) |
|---|---|---|---|---|---|
| 1 | −747.271 × 10–3 | 802.018 × 10–9 | 501.958 × 10–3 | 0.1154 × 106 | 371.012 × 10–3 |
| 2 | −793.46 × 10–3 | 803.298 × 10–9 | 37.491 × 10–3 | 0.0184 × 106 | 376.892 × 10–3 |
| 3 | −502.782 × 10–3 | 26839 × 10–9 | 103.324 × 10–3 | 0.0010 × 106 | 170.134 × 10–3 |
| 4 | −789.093 × 10–3 | 3505 × 10–9 | 36.365 × 10–3 | 0.0041 × 106 | 433.538 × 10–3 |
4. Conclusions
From the results obtained in this study, it can be concluded that a molten, ternary mixture of 80% wt. AlCl3, 10% wt. NaCl, and 10% wt. KCl along with a small amount of KI as additive is a suitable electrolyte bath for electrolytic plating of aluminum on mild steel. Parameters such as current density and electrodeposition time have a significant effect on the coating morphology. The best coating quality was obtained at the deposition temperature of 175 °C at current density of 0.022 A.cm−2 during 60 min. Under these conditions, silvery, bright, and finely crystallized aluminum coating with good adhesion could be deposited. At lower or higher current density, spongy or dendritic deposition occurred. In times less than 60 min, the formation of coatings could not be completed. Annealing the samples formed brittle intermetallic layers that contained a large fraction of Fe2Al5; the apparent activation energy of formation of the Fe-Al intermetallic layer was calculated as 74.2 kJ.mol−1 from the growth kinetic constant. Potentiodynamic polarization tests showed that the aluminum-coated samples exhibit lower corrosion rates than the bare substrate, showing that such coatings can successfully be used to protect mild steel from corrosion. The observations in this study showed that the employed method can be considered as an efficient, inexpensive means of protecting steel from corrosion in corrosive media by forming a dense layer of aluminum on the substrate.













