Abstract
Polydopamine (PDA) is a mussel-inspired and a melanin-mimicking material that has attracted considerable attention during the recent years. This 'polymer' displays diverse promising properties, like its simple preparation procedures, easy functionalization, free radicals scavenging activity, outstanding photothermal and photoacoustic performance, and its great biocompatibility and biodegradability. A remarkable feature of PDA is its ability to form colloidal nanosized particles or nanoscaled coatings, allowing the preparation of various nanoparticulate structures. The first studies into PDA mainly explored the polymerization mechanisms of this material and the development of controlled preparation protocols. Later works focused on the investigation of these nanomaterials for the design and development of multifunctional platforms and their implementation in multiple biomedical fields, particularly in cancer treatment and bio-imaging. The purpose of this review is to (a) give a detailed overview about the synthesis methods of PDA and the formation mechanisms proposed so far in the literature, (b) outline the remarkable physico-chemical and functional properties of PDA nanomaterials, and (c) summarize the application of PDA-derived nanosystems in cancer theranostics and particularly in drug delivery and light-mediated cancer therapy with a special emphasis on the different strategies that can be used for the design of smart nanosystems with bimodal photothermal/photodynamic properties. Finally, a comparison of physicochemical properties and biomedical applications between PDA and other catecholamine derivatives is made.
Export citation and abstract BibTeX RIS
1. Introduction
The development of bioinspired and bio-mimicking materials has become a predominant strategy for the design of novel nanomaterials with interesting potential functions and promising biomedical applications such as for the treatment of cancer [1, 2] and bacterial infections [3]. To this end, melanin pigments have gained growing interest among the bioinspired materials for the design of multifunctional nanomaterials for many biomedical applications. In fact, melanin pigments are ubiquitous natural biopolymers of dark color that can be found in many types of living organisms ranging from bacteria [4] and fungi [5] to plants [6] and mammals [7]. The key enzyme of melanogenesis in many microorganisms and animals is the tyrosinase [8]. While these pigments contribute to the pathogenesis of some microorganisms and to the seeds color in plants, they play different physiological roles in mammals. In these latter species, melanin pigments can be found in various parts of living organisms such as skin, eye, hair and brain medulla, and can be divided into different groups depending on their origin and location, including eumelanin, pheomelanin, neuromelanin, allomelanin, etc [9, 10]. Due to their intrinsic properties, natural melanin pigments and their vesicles have been extracted from natural sources such as cuttlefish ink [11], hair [12], and skin [13], however due to the complicated extraction and purification procedures, synthetic methods of melanin derivatives were required.
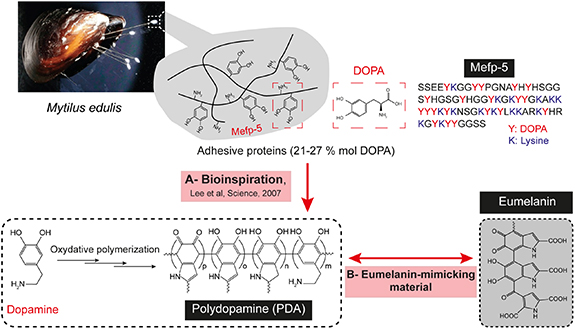
On another hand, the astonishingly strong adhesion of marine mussels, Mytilus edulis, to diverse wet surfaces has been a great inspiration for the design of new biomaterials for surface coating [14–17]. This mussel unique robust adhesion was long investigated and was ascribed to the Mytilus edulis foot proteins (Mefps), particularly Mefp-5, which is found preferentially near the adhesive plaque/substrate interface and is able to glue surfaces with a high strength and stability [18]. These Mefps were found to be rich in 3,4-dihydroxyphenylalanine (DOPA) and lysine residues [19–21]. Inspired by these proteins' chemistry, Messersmith's group reported that, under mild alkaline conditions, dopamine hydrochloride can spontaneously undergo an oxidative self-polymerization process to afford a strong black-colored coating material, called 'polydopamine' (PDA), able to adhere to the surface of all tested inorganic and organic substrates (figure 1(A)) [18]. Indeed, dopamine is a DOPA derivative catecholamine bearing both catechol and amine functions and represents hence a powerful building block for the elaboration of Mefps mimics.
Figure 1. Polydopamine, a nature-inspired polymer, (A) the molecular composition of Mytilus edulis foot proteins rich in DOPA and lysine amino acids inspired the preparation of a strongly adhesive coating material, polydopamine, obtained from dopamine oxidation, (B) the oxidative polymerization of dopamine leads to a eumelanin-mimicking structure.
Download figure:
Standard image High-resolution imageThe dopamine auto-oxidation was investigated previously by Swan et al in 1970 for the synthesis of a 'dopamine-melanin' material in studies related to the chemistry of melanins [22]. However, since the pioneering report by Lee et al, PDA has mainly emerged as an attractive organic coating material, superior to the common surface modification strategies like layer-by-layer assembly or self-assembled monolayers [23, 24]. Interestingly, owing to the abundant nucleophilic and electrophilic reactive sites [25], PDA coatings were proven able to undergo secondary reactions, offering thus an interesting platform for the immobilization of a large repertoire of molecules on the PDA -modified substrates [18]. Hence, PDA served as a versatile surface coating and functionalization material in diverse material science fields and allowed the development of diverse PDA -coated nanostructures including multifunctional core@shell nanocomposites and hollow nanocapsules or nanotubes [23, 26–28].
Additionally, as it shares the same precursor molecules, synthesis mechanisms and similar structural and physicochemical properties with the natural eumelanin pigment, PDA is also regarded as a synthetic analogue of naturally occurring melanin (figure 1(B)). Actually, the oxidative process yielding PDA coatings leads simultaneously to the formation of aggregates in the bulk solution. Efforts have been devoted to the valorization of such aggregates and the control of PDA growth processes in order to obtain stable nano-colloids, that can be used as melanin-like nanoparticles.
Eumelanin, a brown-black melanin derived from Tyrosine oxidation into L-DOPA displays a number of intriguing physico-chemical properties at the origin of their important roles in diverse biochemical processes and biological activities. For instance, owing to its broad absorption spectra covering the entire UV-visible-NIR regions, its radical scavenging effect, redox active metal ions chelation and anti-oxidant ability, its fast non-radiative relaxation process and so forth, eumelanin exhibits important photo-protective and anti-oxidative functions and contributes to the homeostasis of several biochemical systems [9]. Importantly, eumelanin is biologically degradable into non-toxic metabolites. These inherent properties are closely related to their chemical composition and molecular buildup. Similarly, owing to its outstanding physico-chemical properties, PDA eumelanin-like nanoparticles emerged as powerful biocompatible and biodegradable nanoplatforms with interesting properties such as anti-oxidant effects, photothermal conversion ability and photoacoustic properties [26, 29–31].
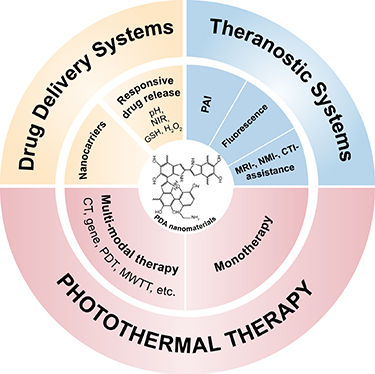
Altogether, due to the advantageous concomitant features derived from both mussel- and melanin-mimicking properties, PDA has attracted extensive attention for the design of novel smart nanoplatforms with a plethora of possible applications including in energy and environmental materials sciences but particularly in the biomedical field where it emerged as a material of choice for the elaboration of diverse multifunctional nanosystems of great potential in fields like cancer therapy (figure 2), infections treatment, biosensing and bioimaging, tissue engineering, etc.
Figure 2. Applications of polydopamine nanomaterials in cancer therapeutic/theranostic fields.
Download figure:
Standard image High-resolution imageExcellent reviews have been published in recent years regarding PDA -based nanomaterials with a great focus on the achievements in PDA-derived platforms development and their applications in different domains [26, 29–36]. However, recent comprehensive reviews about the physico-chemical prospects governing the formation, behavior and functions of PDA nanomaterials are still lacking. Therefore, in this review, we will outline the significant known features and bridge them with the recent advancement in the understanding of the structure–properties–processing relationships. Throughout this review, the discussion will only concern PDA-based particulate nanosystems, i.e. PDA nanospheres, PDA nanocapsules, and PDA-coated nanosystems. First, we will describe the preparation methods reported for the fabrication of PDA products and the major key factors allowing to elaborate PDA colloidal structures and shells of controlled properties. Next, we will overview the most relevant structural models proposed, to date, for PDA and the underlying formation mechanisms. Thereafter, we will discuss the fundamental physico-chemical properties of PDA nanomaterials and elucidate their direct implementation in cancer treatment and/or diagnosis, in particular the drug delivery of anticancerous molecules and the photo-based cancer therapies mainly photothermal therapy (PTT) and photodynamic therapy (PDT). Analogies between PDA and eumelanin will be made in the different sections of this review to give a general understanding of PDA properties.
2. Preparation strategies of PDA-based materials
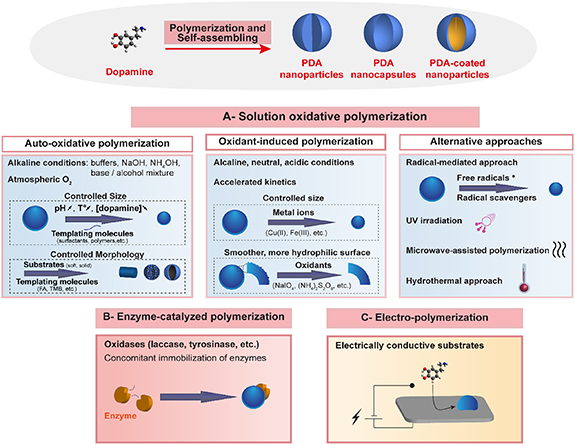
The preparation methods of PDA nanomaterials have largely evolved from the first protocol described by Messersmith et al [18]. Generally, in presence of a substrate, with dimensions down to the nanoscale, a thin PDA layer can be deposited overtime on the substrate's surface, via the oxidation of dopamine precursor. Simultaneously, insoluble PDA aggregates are formed in the medium and continue to grow into particles. Many progresses have been achieved in the comprehension of the mechanisms governing PDA growth and its aggregation processes. In addition, several efforts have been devoted in the aim of expanding the synthetic toolbox of PDA materials and adjusting the set of experimental parameters for the formation of PDA shells or particles with strictly controlled morphological and physico-chemical properties. In this section, we will describe the most reported strategies applied to control PDA synthesis, particularly PDA nanocolloids and PDA-coated nanosystems, summarized in table 1 . They are commonly categorized into three major approaches, namely the solution oxidation (figure 3(A)), the enzymatic oxidation (figure 3(B)) and the electropolymerization (figure 3(C)) of dopamine monomers. We will discuss the different key parameters shown to have a great impact on the dopamine oxidation kinetics and consequently on the prepared PDA nanomaterials.
Figure 3. Illustration of the preparation methods used for polydopamine synthesis: (A) solution oxidation of dopamine and typical parameters and additives controlling dopamine oxidation and polymerization rates, and the structural characteristics of nanoparticles size and coatings deposition, (B) enzyme-catalyzed oxidation of dopamine following a melanin biosynthesis-mimicking approach, (C) anodic oxidation of dopamine. FA: folic acid, TMB:1,3,5-trimethylbenzene.
Download figure:
Standard image High-resolution imageTable 1. The major methods used for the preparation of controlled polydopamine particulate nanostructures.
| Preparation method | Obtained nanostructures | Impact on nanostructures properties | Ref | |
|---|---|---|---|---|
| Auto-oxidative solution polymerization | Basic buffers (Tris, Phosphate, Bicarbonate), in the presence of substrates | Preparation of PDA-coated structures Few nanometers to few hundreds of nanometers | Tris buffer is the most used basic buffer Choice of buffer impacts the polymerization kinetics and thickness, roughness, and paramagnetic properties of PDA layer | [43] |
| Sodium hydroxide/water | Preparation of PDA nanospheresFew tens to few hundreds of nanometers | Hydroxide ions concentration impacts the size of PDA nanoparticles. | [37, 40, 45] | |
| Ammonium hydroxide/water/ethanol | Preparation of monodisperse PDA nanospheres Few tens to few hundreds of nanometers | Ammonia concentration impacts the size of PDA nanoparticlesEthanol used at a volume fraction of 25% to 40% to obtain monodisperse nanoparticles | [46, 49] | |
Reverse microemulsion:
| Preparation of small PDA nanospheresFew tens of nanometers | The size of dispersed ammonia droplets impacts the size of PDA nanoparticles | [54] | |
| Basic conditions, in the presence of templating molecules (polymers, polyelectrolytes, surfactants, proteins, etc) | Preparation of small PDA nanospheres Nanometer to few tens of nanometers | Templates act as confined environments for PDA synthesis Templates concentration impacts the size of PDA nanoparticles | [55, 56, 58] | |
| Basic conditions, in the presence of folic acid (FA) | Preparation of PDA nanospheres/nanotubesFew tens to few hundreds of nanometers | FA and dopamine concentrations impact the morphology of PDA nanostructures | [61, 112] | |
| Basic conditions (mainly buffers), in the presence of core templates (soft templates or solid templates selectively removed) | Preparation of PDA nanocapsulesFew tens to few hundreds of nanometers | The size and shape of the cores define the size and shape of the hollow PDA structures | [70, 73, 75, 76, 113] | |
| Chemically assisted solution oxidative polymerization | Acidic, neutral or basic conditions, in the presence of oxidants (like NaIO4) metal ions (like Cu (II)) | Preparation of polydopamine nanoparticles and PDA-coated structures Few nanometers to few hundreds of nanometers | Choice of oxidant type and concentration impacts the kinetics of PDA polymerizationOxidants and metal ions impact the structure of PDA and the thickness and roughness of the coating Fast polymerization kinetics are obtained in comparison to conventional methods | [80, 84, 85] |
| Basic conditions (mainly ammonia), in the presence of free radicals or radical scavengers | Preparation of PDA nanospheresFew tens to few hundreds of nanometers | These additives allow radical tuning in the solution and a control of PDA polymerization and of the size of PDA nanoparticles | [92] | |
| Enzymatic polymerization | Enzyme-catalyzed dopamine oxidation and polymerization (like laccase) | Preparation of PDA nanoparticles or PDA-coated nanostructuresFew tens to few hundreds of nanometers | Preparation of stable and uniform nanostructures, resembling to melanin materialsPossible simultaneous immobilization of active enzymes on the yielded structures Green products | [99, 101, 102] |
| Electro-polymerization | Anodic oxidation of dopamine | Preparation of PDA-coated nanostructures or PDA nanospheres,Few nanometers to few hundreds of nanometers | Exclusively used to coat conductive nanostructures like TiO2 or Fe3O4Fast polymerization kinetics are obtained in comparison to conventional methods Ultra-small fluorescent PDA nanospheres were obtained by anodic microplasma electrochemistry | [108, 109, 111] |
2.1. Solution dopamine oxidative polymerization
2.1.1. Auto-oxidative polymerization in alkaline conditions
To date, the solution auto-oxidative self-polymerization of dopamine hydrochloride in mild alkaline solution (pH > 7.5) under ambient atmosphere remains the simplest and most common strategy used for the preparation of PDA products, due to its versatility, cost-effectiveness and reproducibility. PDA synthesis occurs rapidly and is accompanied with a color change of the solution from colorless to pale brown turning progressively to dark brown or black.
At first, this method has been described for the preparation of PDA coatings and was achieved in an aqueous Tris buffer solution (pH 8.5) [18]. Soon later, this protocol was further adapted to produce PDA coatings of controlled thicknesses and morphologies and more interestingly to valorize the PDA aggregates formed in the bulk solution. It was proposed that the oxidation of dopamine monomer leads to the formation of a dopamine-quinone moiety, which undergoes cyclization and further oxidation and rearrangement processes, yielding eventually PDA products. Kinetics studies have generally agreed that the first oxidation step of the deprotonated dopamine was the rate-determining step in PDA formation. The following intramolecular cyclization process was relatively rapid. Consequently, several strategies have emerged based on controlling the oxidation reaction kinetics mainly through tailoring the following experimental conditions: the solution pH, the used buffer/solvent, dopamine concentration and the temperature (figure 3(A)).
2.1.1.1. Choice of solvent and pH value
The pH value of the reaction solution is a vital factor allowing to control the kinetics of dopamine auto-oxidative self-polymerization. Based on the generally adopted mechanism of PDA synthesis (scheme 1(A)), a basic pH appears crucial for the consumption of the hydrogen protons produced as PDA formation progresses. This allows the shift of the redox equilibrium towards PDA production [26] and an increase in the initial solution pH leads to the acceleration of dopamine oxidation rates [37, 38]. Hence, by increasing the initial pH, an increase in the thickness of the PDA deposited layer could be achieved in the case of PDA coatings [39], while when producing PDA nanoparticles, a decrease in the particles size could be obtained [40]. The choice of the reactant solvent is another critical factor of a great impact on the outcomes of dopamine oxidative polymerization.
Scheme 1. (A) The generally agreed dopamine auto-oxidation process, leading to the formation of polydopamine building units, (B) structural models proposed for polydopamine generated from covalent polymerization pathways or physical self-assembling processes.
Download figure:
Standard image High-resolution image2.1.1.1.1. Alkaline buffers
To date, the use of Tris buffer (at pH 8.5) as a basic polymerization initiator remains the most common method used for the preparation of PDA coatings [41]. It is worth noting that the structural investigation of the as-prepared coatings revealed that Tris could be incorporated in PDA scaffold via covalent coupling between its primary amine and the dopamine-quinone intermediate, which risks impacting the materials properties [42, 43]. However, Tris incorporation in PDA matrix was found to be prevalent only at the highest Tris/dopamine molar ratios [42]. To avoid such interference, other amine-free alkaline buffers were reported for the preparation of PDA coatings, in particular phosphate and bicarbonate buffers [42–44].
2.1.1.1.2. NaOH solution
To fabricate colloidal PDA nanospheres, further acceleration of the dopamine oxidation kinetics is needed. The synthesis of nano-sized particles was reported for the first time in 2011 by Ju et al [37]. In their protocol, sodium hydroxide (NaOH) aqueous solution was used in replacement to the above-mentioned basic buffers, which affected considerably the oxidation kinetics of deprotonated dopamine and allowed the production of nanoparticles with a size less than 100 nm. This protocol was later adopted by several groups for the synthesis of melanin-mimetic nanospheres [40, 45]. Later, Cho et al investigated more in depth the hydroxide-ion mediated synthesis of stable PDA nanoparticles [38]. Considering their critical role in hydrogen abstraction during dopamine oxidation, it was found that the concentration of hydroxide ions directly influences the initial dopamine oxidation rate which determines the number of nuclei for nanospheres growth and enables the control of nanospheres diameter. Precisely, an increase of hydroxide ions concentration induced a linear acceleration of the oxidation rate and an early saturation of the solution with oxidized dopamine, leading therefore to the formation of more nucleation sites and consequently the production of smaller nanospheres [38]. Moreover, in the used hydroxide concentrations range, nanoparticles surface charge was highly negative (−44 mV) which prohibited particles aggregation and maintained their colloidal stability.
Although sodium hydroxide-mediated protocol was revealed efficient for the preparation of nano-sized PDA spheres with a reproducible fine control over their size and a good colloidal stability in water and biological media, large PDA aggregates were simultaneously produced in the reaction medium, which necessitated an additional time-consuming purification step in order to be removed from the final preparation (by low-speed centrifugation, supplementary filtration, etc) [37, 45]. Relatively high temperatures were also needed to reach the desired nanometric size (around 50 °C).
2.1.1.1.3. Water/organic solvents mixture or organic solvents
Further efforts have been devoted to establishing alternative preparation pathways offering a better control of the nanospheres size distribution. In this context, Lu's group reported for the first time a new protocol allowing the direct synthesis of monodisperse PDA nanospheres with a highly controlled size and morphology, using a water-ethanol mixed solvent (with 29% (v/v) ethanol) in presence of ammonium hydroxide (NH4OH) as the basic catalyst [46, 47]. The size of the as-prepared nanospheres could be tightly tuned in the range of several tens to hundreds of nanometers by varying the ammonia to dopamine molar ratio; higher ratios resulted in smaller nanoparticles. In a parallel study, Yan et al highlighted the important role of water-alcohol mixed systems in the preparation of monodisperse spherical PDA nanoparticles; dopamine oxidation was conducted here in presence of Tris buffer at pH 8.5 and ethanol mixture [48]. Interestingly, it was revealed that pure ethanol could impede dopamine polymerization [48, 49], which suggested that its introduction in the reactant medium as a co-solvent may offer a control over the polymerization rate and consequently over PDA particles growth process. To better explain all these findings, Jiang et al studied the formation of PDA particles achieved in ammonium hydroxide in presence of various alcohol-water mixtures and calculated the Hansen solubility parameter corresponding to each solvent system [49]. Their results demonstrated that the monodisperse PDA nanospheres could only be obtained at an appropriate volume fraction of ethanol ranging from 25% to 40%, which corresponded to the solvent mixture solubilizing at best the dopamine monomer. The highest conversion of dopamine was also obtained in this range of ethanol/water volume ratios. This alcohol-water system was not only limited to ethanol, but other alcohols such as methanol (at 10% or 20%) or 2-propanol (at 40%) were also proven to be efficient in the preparation of spherical monodisperse nanoparticles. Isopropyl alcohol and ethylene glycol were also reported in other studies for PDA nanoparticles preparation and showed similar results [48].
It should be noted that dopamine polymerization could be also achieved in pure organic basic solvents like piperidine, which allowed to expand PDA applications to hydrolysable substrates coating but also to simultaneous immobilization of water-insoluble molecules [50].
2.1.1.2. Dopamine concentration
Dopamine concentration is another key parameter which considerably affects PDA particles morphology and films deposition kinetics and characteristics [39, 42]. Increasing the initial dopamine concentration (from 0.1 to 5 g l−1) resulted in a linear increase of the coating thickness [39, 51], but also in increasing the coatings surface roughness [43]. The dopamine concentration first reported by Lee et al and commonly used for general surface functionalization purposes is of 2 g l−1, however, lower concentrations (<0.5 g l−1) were preconized when PDA is used for the functionalization of nanostructures [51]. In fact, the decrease of dopamine concentration to such low values allowed to effectively reduce the formation of PDA particles and thus their aggregation limiting the inevitable increase of PDA shells roughness [51]. Furthermore, it should be taken into account that dopamine concentration can also impact the molecular composition of the PDA products, in particular the relative proportions of cyclized and uncyclized units. In fact, at very low concentrations of this catecholamine (0.1 g l−1), a larger ratio of cyclized indole units is obtained [42]. Dopamine concentration should also be properly adjusted for the preparation of monodisperse PDA nanoparticles, since unstable small nanospheres were obtained at low dopamine concentrations, while high values led to large nanospheres with an irregular shape [38]. Hence, tailoring dopamine concentration in the range yielding monodisperse nanoparticles allows the control of nanoparticles size and yield [37, 38, 52].
2.1.1.3. Temperature
The experimental temperature is another important factor that has to be carefully adjusted in order to control dopamine oxidation rates. Indeed, increasing the reaction temperature resulted in accelerated PDA deposition rates, leading to thicker and more hydrophilic PDA coatings in comparison to those formed at lower temperatures [53]. On the other hand, in the case of PDA nanoparticles production, increasing the temperature gave rise to smaller nanospheres with higher yields [37, 52], which could be explained by enhanced dopamine oxidation kinetics. Cho et al suggested that the temperature can impact the size of PDA nanospheres via different modalities [38]. At high values, the hydroxide ions concentration would increase as a result of pKwater decrease, which leads to more nucleation sites and eventually smaller nanoparticles.
2.1.1.4. Templates
To date, the traditional template-free synthesis strategy based on the simple dopamine polymerization in alkaline aqueous or water-ethanol solutions remains largely adopted for the preparation of the PDA-based nanoplatforms. However, several templates-guided approaches have been also developed, permitting to further monitor dopamine polymerization and to shape PDA-based nanostructures into colloidal PDA nanoparticles, hollow nanocapsules or tubes, mesoporous nanoparticles, etc.
For instance, a reverse microemulsion-based method was employed to prepare relatively small monodisperse PDA nanospheres with a tunable size ranging from 25 to 43 nm [54]. In this method, ammonium hydroxide was used as the aqueous phase and was added to a mixture of cyclohexane, used as the oil phase, and Igepal CO-520, used as the surfactant. After sonication and stirring, ammonia aqueous nanosized droplets are formed in the oil cyclohexane phase and act as a confined nanoreactor for the synthesis of PDA nanospheres, with a tunable size obtained by tailoring the amount of ammonium hydroxide and the reaction temperature.
Additionally, PDA nanoparticles synthesis could also be controlled through the addition of templating molecules such as surfactants [55], polyelectrolytes [56] and other polymers [57]. Cationic and anionic surfactants (like hexadecyltrimethylammonium bromide and sodium dodecylsulfate (SDS), respectively) [55] allowed the preparation of small nanoparticles with a tunable size that could be considerably decreased by increasing surfactant's concentration. For the highest concentrations of surfactants, exceeding their critical micellar concentrations, nanoparticles could reach a range of sizes only slightly higher than those of surfactants micelles [25]. It was proposed that surfactants interact with dopamine and act as templates for confined PDA growth. The structure of the as-prepared PDA was similar to the conventionally produced PDA. Similar to surfactants, a vast repertoire of polyelectrolytes were found effective in controlling the size of PDA nanospheres in a concentration-dependent manner [56]. It was proposed that the mechanisms by which polyelectrolytes act may differ depending on their chemical nature. As an example, it was suggested that polycations bearing primary amine functions, like poly(allylamine hydrochloride), would interact covalently with the quinone groups of oxidized dopamine units and small PDA aggregates and act as 'capping agents' limiting preferentially PDA formation near the polyelectrolyte and yielding, for sufficiently high polyelectrolyte concentrations, nanoparticles slightly larger than the polyelectrolyte diameter.
Several other templating molecules were reported in the literature for the controlled preparation of PDA-based nanomaterials, including certain proteins like human serum albumin [58] which allowed the increase of dopamine oxidation rates and yielded small stable biocompatible PDA nanoparticles. Interestingly, by screening the proteins of skin melanocytes found usually surrounding eumelanin granules, a specific amino acids diad in proteins, L-lysine (K)–L-glutamic acid (E), was found particularly efficient in the oxidation control process [59, 60]. This KE diad would likely play a templating effect in PDA nanoparticles assembly via establishing synergistic interactions with dopamine (hydrogen bonding and π-cation interactions) leading to an increased residence time of dopamine on this dipeptide as investigated by molecular dynamics simulations [60].
Template-guided dopamine polymerization pathways could also induce significant morphological changes of PDA assemblies. The most cited studies to this regard described the drastic impact of folic acid (FA) on the obtained PDA nanostructure [61, 62]. In fact, when this molecule was added to dopamine solution, new aggregated nanobelts- and nanofibers-type structures were generated in the alkaline solution, preferentially at 60 °C. The authors proposed that the presence of FA favored the formation of porphyrin-like indolic tetramers and enhanced their ordered stacking through π–π stacking interactions, which would lead preferentially to an aggregation into the graphite-like ordered nanostructures. Mesoporous PDA nanostructures could also be designed using triblock copolymer Pluronic F127/1,3,5-trimethylbenzene (TMB) emulsion composites as organic templates in Tris buffer solution [63, 64]. It was proposed that the formation of the mesoporous structure was favored by the π–π stacking interactions between PDA aggregates and the π-electron-rich TMB. Primary PDA particles formed in the solution would diffuse into the organic TMB phase and subsequent packing of PDA particles would occur leading to the mesoporous nanostructure with particles and pores sizes tunable via varying TMB/F127 weight ratios. The preparation of PDA-based Janus composites as multifunctional nanoplatforms was also described in several reports, using diverse innovative strategies [65–68]. In addition, hollow PDA (nano)capsules or (nano)tubes could also be obtained through polymerization of dopamine on soft templates (liquid–liquid interface) like emulsion droplets [69–72] and tetrahydrofuran-Tris buffer mixture [73] or on solid particulate templates of various sizes and shapes (solid–liquid interface) like SiO2, CaCO3, etc, followed by selective removal of the template core [74–78].
2.1.2. Chemically assisted oxidative polymerization of dopamine (using exogenous oxidants)
Despite its versatility and simplicity, the auto-oxidative pH-induced method is a relatively slow process demanding at least several hours to overnight incubation times [79], and requires alkaline conditions in order to reach reasonable reactions rates [80]. Additionally, dissolved atmospheric oxygen is a critical element contributing to dopamine auto-oxidation and polymerization reactions, via hydrogen abstraction [18, 81, 82]. Indeed, when oxygen was eliminated from the solution, either by permanent nitrogen gas bubbling or by deoxygenation under vacuum, the incubation of dopamine in alkaline conditions, even at high pH values, did not visibly lead to PDA production [37].
Therefore, efforts have been made to overcome these limitations and alternative preparation strategies, based on the use of exogenous oxidants, have been developed to accomplish faster oxidation kinetics and further adjust PDA products properties. Such alternatives allowed, potentially, the production of PDA under neutral or acidic pH, which would be particularly interesting to expand PDA surface coating and one-pot drug/adjuvant co-loading applications to base-sensitive or insoluble substrates and molecules [25].
In fact, under high concentrations of oxygen (up to pure oxygen), the reaction kinetics increased drastically and allowed the deposition of highly homogenous and ultra-smooth PDA layers, achieved in much shorter deposition times [83]. Beside pure oxygen, other water-soluble oxidizing reagents were proven efficient to induce accelerated dopamine polymerization and could trigger PDA formation in mild alkaline, in neutral or acidic solutions [24, 41, 81, 84]. In this context, various oxidants have been employed including sodium periodate (NaIO4), ammonium peroxydisulfate ((NH4)2S2O8) and potassium permanganate (KMnO4), in addition to multiple other inorganic chemicals and metal ion additives such as Cu(II), Fe(III) and Mn(III) salts, etc [44, 80, 81, 84–86]. The choice of the used oxidant as well as its concentration has a major impact on the polymerization rates and the pathways favored during PDA assembling process [87]. Besides, mixtures of CuSO4/H2O2 [88], FeCl3/H2O2 [89] or FeSO4/H2O2 [90] were also used as oxidants for ultra-fast PDA shells or nanoparticles formation. Despite the great enhancement of PDA production kinetics, the possible effect of metal ions on the structure and properties of PDA should be taken into account. Indeed, PDA components (particularly catechol groups) exhibit a high affinity and complexation ability towards various metallic cations, leading to the inevitable incorporation of these latter in PDA matrix. Depending on the redox and complexation ability of the metal ion, this would lead to changes in PDA chemical composition (loss of nitrogen content, presence of metal content) and consequently to changes in its physico-chemical properties [24, 85, 91].
2.1.3. Radical-, ionization- and microwave-assisted dopamine polymerization
In order to avoid chemical contamination of PDA nanomaterials encountered when using exogenous oxidants, alternative strategies have been developed to obtain fast dopamine polymerization kinetics, for the formation of PDA under neutral or even acidic conditions. Recently, Wang et al reported a free radical-mediated strategy allowing further control of the size of PDA nanoparticles prepared in a water/ethanol/ammonia mixture, via tuning the free radicals in the reaction medium [92]. Based on the speculation that PDA formation process could involve radical coupling pathways related to the generation of semi-quinone radical intermediates via dismutation reaction [93, 94], two strategies were tested and found effective to control PDA formation. The first one consisted in quenching the radical intermediates generated during PDA formation via the addition of strong radical scavengers (such as edaravone), which leads to the inhibition of nanoparticles growth. Whereas the second strategy was based on the addition of stable free radicals (such as PTIO) which facilitates the seed formation process. Both strategies offer an interesting modality to control PDA nanoparticles diameter, while maintaining the characteristic chemical structure and physico-chemical properties of the conventional nanoparticles.
Free radical species triggering PDA formation can also be generated by providing external energy to the system via UV irradiation [95, 96]. This light-induced method allowed the acceleration of dopamine polymerization under alkaline conditions and was also proven effective for PDA formation at neutral or acidic conditions (down to pH 2.0) [96]. PDA formation could also be achieved by using visible light, under neutral conditions (pH = 7.0), in presence of 9-mesityl-10-methylacridinium ions as a photocatalyst [97]. Excited, this molecule can activate the solution dissolved oxygen, which triggers dopamine polymerization. The microwave-assisted polymerization is another method found to accelerate PDA formation in a chemical-free manner. Using this technique, only 15 min were needed to deposit 18 nm film thickness of PDA under 1000 W versus several hours for the traditional protocol [79]. This enhancement effect of the polymerization kinetics was attributed to an increased radical generation by microwave irradiation and the resulting superheating effect. However, to the best of our knowledge, no previous studies have been reported about using waves-based polymerization processes for the preparation of PDA nanoparticles.
A hydrothermal process was also used to trigger dopamine oxidation and self-polymerization and allowed to extend PDA production to strong acidic environments (pH 1.0 and at 160 °C) [98]. The efficiency of this strategy was attributed to the pronounced auto-ionization of water under the high temperature and pressure employed, leading to the formation of hydroxyl ions in the bulk solution which are involved in dopamine oxidation reactions.
2.2. Enzymatic dopamine polymerization
The process of melanin biosynthesis in living organisms has inspired the development of a new approach for the preparation of PDA nanomaterials based on enzyme-catalyzed dopamine oxidation and self-polymerization. Several enzymes reported for the oxidation of phenolic compounds and aromatic amines have been employed as catalysts to trigger dopamine oxidative processes. For instance, laccase has been employed for the preparation of PDA nanoparticles and coatings (at pH ∼5.5). In comparison to the conventional PDA products, the yielded products were interestingly more stable and uniform [99–101]. Horseradish peroxidase was also efficient for rapid oxidation of dopamine in presence of hydrogen peroxide [102]. Also, using an urease catalytic reaction and based on the basification of the reaction medium coupled to urea hydrolysis, Li et al developed an efficient approach offering control over dopamine polymerization and yielding PDA nanoparticles of tunable sizes that increase linearly with the increase of urea concentration [103].
Although it is relatively more complicated than the conventional dopamine auto-oxidative protocol, the enzyme-catalyzed strategy represents an environmentally friendly method that is highly efficient for the preparation of PDA nanomaterials. Interestingly, being inspired by melanin biosynthesis, PDA produced using this method resembles at best to the naturally occurring melanin [30, 36]. Furthermore, this approach is particularly advantageous for simultaneous immobilization of enzymes with preserved enzymatic activity in a one-pot preparation method.
2.3. Dopamine electro-polymerization strategy
Electropolymerization of dopamine emerged as an alternative approach mainly used for the preparation of PDA coatings [104–107] on diverse conductive nanostructures (TiO2 nanotubes [108], Fe3O4 nanoparticles [109], nanoporous gold film [110], etc) with rapid polymerization and high deposition rates. Also, PDA-coated magnetite nanoparticles could be electro-synthesized in a one-pot reaction. By varying the time at which dopamine is added to the reaction medium, it was possible to yield nanocomposites with controlled nanoparticles sizes and shell thicknesses [109]. Interestingly, uniform fluorescent PDA nanoparticles were obtained by Wang et al using a novel anodic microplasma electrochemistry method. This technique induced the generation of oxidative species that could trigger the nucleation of PDA nanoparticles at the plasma–liquid interface. As the process progressed, the solution pH shifted to acidic values (pH ∼5) which inhibited the further growth of nanoparticles and allowed the control of their size [111].
Despite its simplicity, efficiency and possibility to be performed in a deoxygenated medium, the main drawback of the electro-polymerization method is that PDA production can only occur on electrically conductive substrates, thus limiting its extent of applications.
To summarize, a wide variety of approaches have been established, to date, for a well-controlled preparation of PDA nanomaterials. However, the exact structure and formation mechanisms of PDA are still not fully clear, as will be discussed in the following section.
3. PDA structural models and formation mechanisms
Since PDA discovery, numerous studies devoted to the investigation of PDA molecular structure and its buildup mechanism have been reported [42, 114–119]. However, despite the great efforts invested, the detailed chemical composition of PDA is not fully understood yet and the elucidation of a definite structural model is still a long-lasting debate that has not yet come to its end. In fact, PDA's insoluble nature in common aqueous and organic solvents [114, 115, 119] made it difficult to investigate its structure by means of conventional analytical techniques usually applied for polymers molecular weights characterization [119, 120]. Besides, it is accepted that PDA composition has a marked chemical heterogeneity and can depend on the preparation method and experimental conditions, as explained above [42, 44], which makes the elucidation of an exact structure more challenging.
Various techniques were applied to uncover PDA composition, including mainly mass spectroscopic analyses, solid-state nuclear magnetic resonance (ss NMR), x-ray photoelectron spectroscopy (XPS), powder x-ray diffraction (PXRD), and Fourier transform infrared (FTIR) spectroscopy along with other analytical methods. Theoretical approaches based on molecular energies calculations were also employed in order to provide more insights into PDA molecular organization [115, 118, 121]. Moreover, as previously stated, PDA is commonly considered as a eumelanin-like material and there is a general agreement that PDA shares formation steps with eumelanin biosynthesis. Therefore, comparative structural studies were also undertaken to help bring more understanding of PDA characteristic molecular features [42, 121, 122].
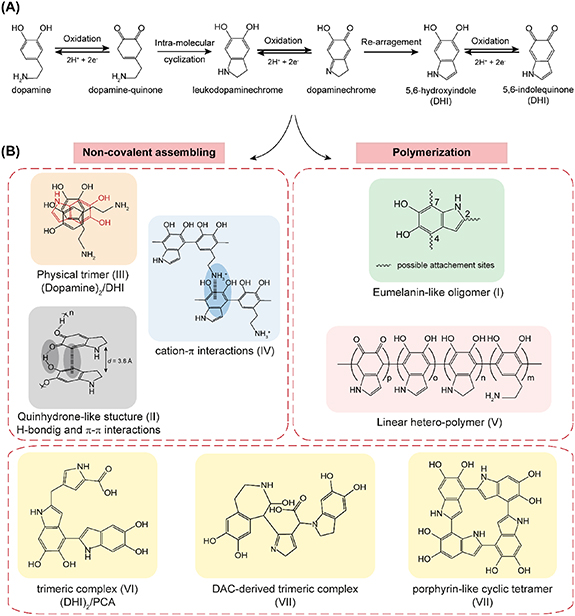
Altogether, structural investigations carried out on PDA products (particles and/or coatings) led to controversial models that fall into three main hypotheses. The first one holds that PDA has a polymeric nature wherein dopamine and/or its oxidation derivatives are covalently linked via aryl-aryl coupling. The second theory suggests a supramolecular assembly arising from three-dimensional association of monomeric units held together via weak interactions such as π–π stacking, Hydrogen bonding (H-bonding), charge transfer or π-cation interactions. The third structural model on the other hand is based on the contribution of both covalent and non-covalent interactions occurring at different stages of the polymerization process and leading to the black insoluble PDA products.
3.1. Covalent polymerization
While introducing PDA, Messersmith's group proposed a chemical structure and a formation mechanism on the basis of a time-of-flight secondary ion mass spectrometry (TOF-SIMS) and XPS data [18]. A major mass peak was detected at m/z 445 and was attributed to a trimer of 5,6-dihydroxyindole (DHI). This trimer was suggested to originate from the fragmentation of a long-chain polymer of similar composition, which led the authors to suggest that PDA is a polymeric material arising from a covalent association of DHI units via biphenyl-type covalent bonds (scheme 1(B), structure I). Based on this observation, a polymerization mechanism similar to that of eumelanin biosynthesis was proposed, wherein the initial driving force consists in the oxidation of dopamine monomer under oxidative alkaline conditions leading to dopamine-quinone formation. This component undergoes then an intra-molecular cyclization (by 1,4 Michael-type addition of the amino group to the phenyl-quinone system) which generates a leukodopaminechrome moiety whose further oxidation and rearrangement leads to the formation of the 5,6-dihydroxyindole. The further oxidation of DHI units generates 5,6-indolequinone and induces spontaneous inter-molecular cross-linkages by dehydrogenative C–C bond formation at positions 2, 4 and 7 of the indole moieties, allowing to yield the black insoluble eumelanin-like PDA polymer [18].
However, this polymerization process known as eumelanin-like poly(indole) structural model became later on an active area of investigation. In fact, chemical differences were noticed when PDA products were compared to pure DHI-polymer (generated from DHI units as exclusive starting monomers) [42, 123]. Furthermore, mass spectroscopic analyses performed by other groups [123–126] on similarly prepared PDA materials did not reveal the presence of the peak at m/z 445 reported by Messersmith's group, and the wide set of analytical methods employed revealed the existence of diverse functional groups referring to the contribution of various moieties as key structural units in PDA buildup. For instance, clear experimental evidence about the presence of primary amines, quinone moieties or carboxylic groups has been widely reported throughout the literature [42, 115, 118]. These observations indicated that PDA would not be solely made of polymerized DHI units as proposed. Indeed, since the overall process of dopamine conversion to DHI was demonstrated to be relatively slow [123, 127], dopamine monomer and the various intermediates derived from its oxidation process are very likely to be simultaneously present in the reaction solution. This would suggest that multiple synthesis pathways involving various kinetics key control points and different intermediates and types of connections (covalent or physical) may occur and lead to a more heterogeneous composition for PDA materials.
3.2. Non-covalent self-assembling
In this context, a different perspective of PDA formation was advanced by Dreyer et al [114] who highlighted the important contribution of non-covalent interactions in this process. They postulated that PDA consists in a supramolecular aggregate of monomers lacking any covalent bond. The monomeric building units, consisting here in 5,6-dihydroxyindoline and its dione derivative primarily, are entirely held together through a combination of H-bonding, π–π stacking and charge transfer complexation. In fact, a detailed study conducted on PDA aggregates, using a variety of solid-state spectroscopic and crystallographic techniques, indicated the presence of these cyclized nitrogenous indoline-type species where the carbon atoms in positions 4 and 7 of the aryl core were rather tertiary (hydrogenated). This observation ruled out the covalent model suggested by Lee et al [18]. Alternatively, Dreyer et al proposed a quinhydrone-like assembly that is further connected via π–π stacking favored by the d-spacing of 3.8 Å consistent with that observed in other π-stacked materials [114, 128]. Such spacing was found to likely facilitate or result from charge transfer between the faces of PDA building units (scheme 1(B), structure II).
Another entirely physical structure was identified by Hong et al wherein dopamine units interfere with DHI moieties to build a supramolecular self-assembled block as a significant component trapped in PDA network [115]. In fact, the authors could identify a robust physically self-assembled [(dopamine)2/DHI] trimeric complex entirely arising from non-covalent interactions probably including H-bonding and π–π interactions (scheme 1(B), structure III). The detection of all the protons from dopamine and DHI species in 1H ss NMR spectra confirmed the non-covalent character of the interactions involved in this complex. However, it was observed that the dissociation of this complex leads to covalent bond-forming oxidation reactions resulting in the formation of a 2,2'-linked DHI-DHI dimer which eventually reacts with dopamine to form a covalent dopamine-DHI-DHI oligomer (C5 of dopamine connected to C4 of DHI). Thus, the authors suggested an overall mechanism for PDA formation implying two different pathways, (a) the self-assembly of dopamine and DHI to generate the physical trimer [(dopamine)2/DHI], along with (b) covalent bonds formation yielding structures like dopamine-DHI-DHI conjugates. More recently, these authors have reported the first experimental evidence that cation-π, a non-covalent interactions mode unexplored so far in PDA materials, can be the driving force responsible for oligomers molecular assembly into PDA granules [116]. Such interactions were demonstrated to occur between protonated amines of uncyclized dopamine moieties and the π-system of indole species leading to highly cohesive PDA structure (scheme 1(B), structure IV).
3.3. Co-contribution of covalent polymerization and non-covalent self-assembling
The aforementioned studies, among multiple others, highlighted that non-covalent interactions would play an important role in PDA assembling. So far, the general consensus favors the contribution of oxidative polymerization leading to covalent oligomers with different molecular weights, as well as the non-covalent interactions probably taking place in the latest stages of PDA synthesis, to yield the final insoluble PDA product. One of the most accepted models concurring with this theory, is the one illustrated by Liebscher et al [118] who re-examined the major structural features present in PDA, using an association of a broad range of analytical techniques, including 1H and 13C ss NMR under magic angle spinning (MAS) conditions, electrospray ionization high-resolution mass spectrometry (ES-HRMS) in addition to XPS and FTIR. Their spectra interpretation revealed the presence of indolic moieties along with open-chain aminoethyl containing compounds and enabled the assignment of each C-atom to a moiety to which it belongs. Interestingly, it was observed that the two carbon lines at positions 4 and 7 cannot both be protonated, which was in disagreement with what was postulated by Dreyer et al [114]. In matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS), the peaks detected were attributed to oligomers composed of uncyclized dopamine and DHI-related units present under different degrees of (un)saturation and/or oxidation states, which concurred with the NMR and XPS results. The covalent connections between building units were proposed to occur via the benzo moieties yielding a linear hetero-polymer (scheme 1(B), structure V). In this model, the contribution of weak interactions was not excluded and was proposed to give rise to a supramolecular assembly of the linear PDA chains. Additionally, their DFT results implied a linear oligomeric chain structure for PDA and the stacking between PDA chains was revealed thermodynamically favorable and H-bonds were very likely to occur. Very recently, evidence for the PDA polymeric nature was provided by Messersmith's group [119]. By means of single molecule force spectroscopy (SMFS), the authors could demonstrate directly, for the first time, the presence of high molecular weight (HMW) polymer chains. So far, the mass spectrometry methods allowed the identification of low molecular weight (LMW) oligomers (up to the octamer level) [118, 120, 124, 125]. The SMFS technique, which enables the study of covalent and non-covalent interactions occurring at the scale of an individual macromolecule, was employed in this study to investigate PDA intermolecular and intramolecular interactions. PDA-coated AFM cantilevers were approached to then retracted from PDA coated or uncoated substrates. The analysis of the force–distance curves obtained revealed the existence of constant force plateaus separated by 'steps'. The plateaus reflected the entropic resistance to polymer chain extension originating from a single molecule stretching event and the steps were related to the rupture of the connections in one or more PDA chains. Steps heights between the plateaus corresponded to the magnitude of the interaction's force. By analyzing these plateaus lengths, the authors could investigate PDA chains lengths and their molecular weights. Their results corresponded to a polydisperse polymer having an average molecular weight value of 11.2 kDa with some polymer chains having above 50 kDa. The great resistance of PDA polymer to very high forces that exceed the threshold of rupture for non-covalent interactions was consistent with a polymeric nature where dopamine and its oxidation derivatives are linked together linearly via aryl-aryl covalent coupling, in a manner similar to that proposed by Liebscher et al [118]. However, the authors do not rule out the presence of small oligomeric species in PDA nanomaterials and stated the possible contribution of weak intermolecular interactions between PDA molecules to yield the final PDA materials.
Non-linear covalent oligomeric blocks and other building units were also proposed in PDA formation. For instance, beside phenylethylamines, indoles and indolines species listed so far, two pyrrolecarboxylic acids (PCAs) were recognized for the first time by Della Vecchia et al as building units of the PDA matrix [42]. The authors conducted a set of chemical and spectroscopic experiments, which enabled them to conclude that PDA is rather a mixture of oligomeric building blocks, up to the tetramer level, composed of three main species: uncyclized dopamine or its quinone-derivative monomers, DHI units and PCA moieties. These latter are likely to derive from partial oxidative cleavage of DHI units under the auto-oxidative conditions related to PDA formation process.
Similarly, Ding et al [125] indicated the presence of a major mass peak at m/z 402, identified as a trimer complex consisting of two DHI units and one PCA moiety [(DHI)2/PCA] (scheme 1(B), structure VI); further covalent linkage between these trimers was found to be unlikely. More recently, Luy et al [124] speculated that the peak at m/z 402 would correspond to a covalent trimer composed of one dopaminechrome (DAC), one DAC degraded unit (2H-pyrrole moiety) and one benzazepine moiety (scheme 1(B), structure VII). It could be noticed that such complex resembles to the dopamine/DHI/PCA-based models reported in the literature [42, 125] and may share physico-chemical properties, allowing the authors to confirm its likelihood. All these authors proposed that covalent interactions occur at the initial stages of the polymerization process, leading to these small blocks, which interact in later stages via non-covalent interactions to build up the supramolecular structure of PDA.
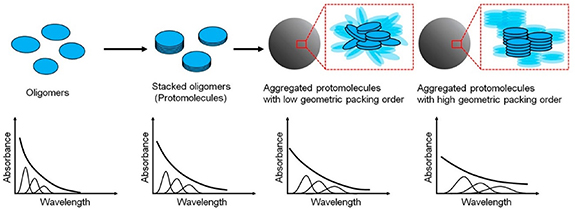
In line with the previous findings, Warren's group studied by means of pump-probe microscopy the role of aggregation in PDA nanoparticles assembly [129] and suggested that PDA nanostructures result from the formation of covalent fundamental oligomeric blocks that stack together via non-covalent interactions, mainly π–π stacking, to form stacked oligomers, also known as 'protomolecules'. These stacked oligomers would further aggregate leading to the hierarchical self-assembled structure of PDA particles.
Multiple other scenarios regarding dopamine polymerization were proposed in the literature, sometimes without sufficient experimental evidence. As an example, porphyrin-like cyclic tetramers for example can result from oxidative polymerization of DHI units and were indeed found in eumelanin materials (scheme 1(B), structure VIII) [126]. Such compounds were identified as the most stable tetramer generated from oxidized DHI units using the computational structural investigation elaborated by Chen et al [121].
To summarize, numerous models have emerged concerning PDA key components and its buildup mechanisms. However, it is currently agreed that these models should not be considered as mutually exclusive, since PDA is very likely to result from the combination of various mechanisms occurring at different stages of the polymerization process, as stated earlier.
4. Physico-chemical properties of PDA nanostructures
PDA products display multiple characteristic features arising from their chemical and structural resemblance to naturally occurring eumelanin. The investigation and interpretation of the physico-chemical and functional properties of PDA materials were indeed often made in analogy to their natural counterparts to allow a better understanding of their common features. It is important to note however, that PDA nanomaterials properties are highly dependent on their preparation method and may particularly vary when polymerization is achieved in presence of additives that risk interacting with or be entrapped within PDA matrix.
4.1. Nanomechanical properties
Nanomechanical properties of biomaterials and drug delivery systems are key parameters that influence their biological performance. In particular, nanoparticles elasticity has been recognized to play an essential role in directing nanoparticles biodistribution through impacting their blood circulation, tissue penetration, cell internalization, etc [130–132]. The evaluation of nanoparticles mechanics is also of great interest for the understanding of their structural and functional properties. However, in the case of PDA nanomaterials, only few studies have dealt with the evaluation of PDA mechanical behavior, but were limited to PDA films and coatings. Mechanical studies performed on conventional PDA coatings revealed relatively high Young's modulus values of few GPa (∼2 GPa to ∼4 GPa), as measured via AFM nanoindentation or compressive film buckling experiments [133–135]. Such elasticity can reflect the good cohesion of PDA nanostructures and the ultra-stability of PDA coatings in vivo. Films elasticity was also investigated using computational approaches. DFT simulations using models based on dimers of dopamine, dopaminochrome and DHI units predicted Young's modulus values ranging from 0.33 to 1.24 GPa, depending on molecules directions [133], while other in silico models generated from controllable covalent cross-linking of DHI units provided Young's moduli comprised between 4.1 and 4.4 GPa for the highest covalently cross-linked polymer model (70%) [135]. Interestingly, increased elastic moduli could be obtained by increasing the inter-unit bonding in silico, suggesting that it is possible to enhance the mechanical strength of PDA materials through increasing the polymerization extent. Indeed, knowing that ∼20% of PDA structure is composed of unpolymerized monomers or partially polymerized oligomers, several relevant experimental studies demonstrated that increased robustness and enhanced mechanical performance of PDA coatings could be achieved through techniques allowing the enhancement of PDA cross-linking level and impacting eventually its cohesive structure. For example, the addition of copper ions [134], calcium cations or cross-linkers like genipin [133], heat treatment (up to 600 °C) inducing PDA carbonization [134], or thermal annealing at moderate temperatures (∼130 °C) [136] resulted in increased Young's modulus values and allowed the production of highly stable PDA coatings resistant to delamination and dissolution, usually occurring under strong alkaline conditions for example. Controlling the experimental conditions of PDA synthesis and post-processing methods has thus a great impact on the mechanical properties of PDA materials and consequently on their stability and biological fate, but would considerably impact their physico-chemical properties.
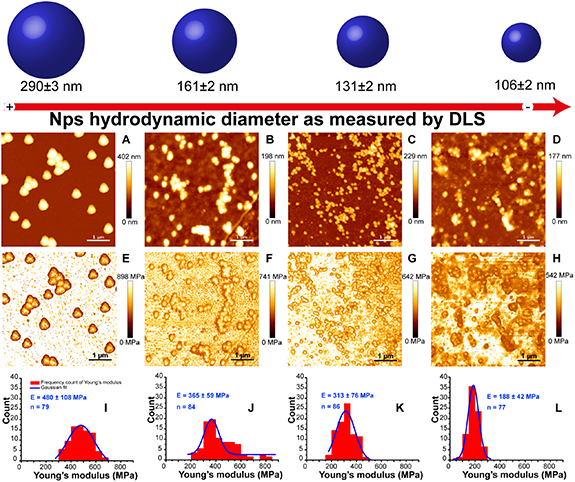
Since it is generally accepted that polymeric nanomaterials would behave differently from their bulk counterparts, the investigation of the nanomechanical properties of PDA nanoparticles is of great interest for a better comprehension of their structural characteristics, but the first report investigating PDA nanoparticles nanomechanical properties has only been published very recently by our group (figure 4) [52]. In this study, we have evaluated the elasticity of PDA nanospheres and examined the impact of nanoparticles size on their Young's modulus, using AFM nanoindentation method. Interestingly, we found that the elastic modulus exhibited by PDA nanoparticles depended on their size, with average values of 188 ± 42 MPa, 313 ± 76 MPa, 365 ± 59 MPa and 480 ± 108 MPa for nanoparticles having average hydrodynamic diameters of 106 ± 2 nm, 131 ± 2 nm, 161 ± 2 nm and 290 ± 2 nm, respectively. The increased elasticity obtained when the nanoparticles size increases could be interpreted by the geometric packing order model described in details in the section 4.4. Indeed, based on this model and on pump-probe microscopy analyses, Warren's group [129] suggested that bigger PDA nanoparticles would have higher geometric packing order between the stacked oligomers forming the core of the nanoparticles, which could explain the higher Young's modulus obtained for bigger nanoparticles in our study. Compared to other polymeric nanosystems such as PMMA, PS and PLGA nanoparticles, PDA nanoparticles showed Young's moduli of about one order of magnitude lower. This difference may be attributed to the different structures, intra-particulate interactions and assembly modes, and would be expected to correlate with a better biological performance, owing to less macrophages uptake and greater tumor accumulation [131, 132].
Figure 4. Nanomechanical study performed on PDA nanoparticles, (A)–(D) AFM height images in Tris buffer of covalently attached PDA NPs with different sizes on silanized mica substrates with their corresponding Young's modulus maps (E)–(H) obtained with JPK Quantitative imaging (QI) mode at a force setpoint of 35 nN and an indentation speed of 50 µm s−1, (I)–(L) represent the histograms distribution of the Young's modulus of NPs with Gaussian fits [52]. Reproduced from [52] with permission of The Royal Society of Chemistry.
Download figure:
Standard image High-resolution image4.2. PDA chemical reactivity
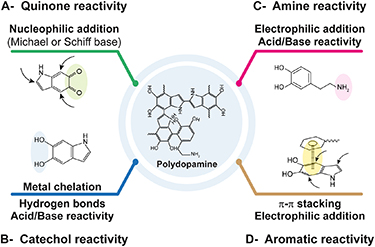
As detailed in section 3, PDA is composed of heterogenous building units, which expose various reactive sites prone to a wide set of covalent, non-covalent and coordinate couplings with different types of materials and molecules (figure 5) [23]. The catechol and amine functions, abundant in PDA, have been recognized as key features responsible for the high reactivity and strong adhesion of this material [137]. The major interactions reported for PDA adhesion mechanism and its reactivity will be discussed below.
Figure 5. Most common interactions (covalent, non-covalent and coordination) involved in polydopamine functionalization.
Download figure:
Standard image High-resolution image4.2.1. Adhesive properties
PDA is the first reported material that can strongly adhere to virtually all types, shapes and sizes of substrates, including noble metals, oxides, polymers, semiconductors, ceramics, low energy surface materials and superhydrophobic surfaces, etc [18, 23]. It can interestingly form a firmly adherent layer likely owing to numerous extremely close anchoring points, which can be used to coat porous materials including metal organic frameworks (MOFs) and mesoporous nanostructures usually difficult to coat [24].
This strong interfacial adhesion allowed the design of diverse core@PDA shell nanocomposites (inorganic or organic nanoparticles, liposomes, etc) in which the PDA layer, highly stable in vivo, can be used to enhance biomaterials biocompatibility, serve as a bridge for secondary functionalization steps, or also be used for its therapeutic or theranostic potential as will be described later. Table 2 illustrates few examples of PDA-coated nanoparticles applied in cancer therapy and the role of PDA shell in the cited systems.
This unique property is most likely the result of an interplay of chemical interactions involving two vital motifs present in PDA, i.e. catechol and amine functions, which represent also the major functionalities present in mussel adhesive proteins [91, 148]. Various covalent and non-covalent interactions can be engaged depending on the surface to coat. The catechol function is able to interact with a wide variety of substrates mainly via coordination bonding, bidentate chelating or bridged bonding and hydrogen bonding, etc [27, 149]. More recently, the primary amine group has gained increasing attraction in the understanding of PDA strong adhesion. The vital role of primary amines was highlighted by Klosterman et al who revealed that the addition of primary amines while forming PDA coatings increased this latter's adhesion properties [133]. Another evidence was brought by the observations revealing that mature particles do not exhibit a high adhesion capacity to surfaces, which was ascribed to the fewer amount of primary amines [25, 42], in comparison to the strong adhesion obtained when surface coating is performed during the early stages of PDA formation [25, 42]. Besides, it was found that poly(catecholamine) coatings exhibited a significantly higher adhesive strength (30 times) than that of poly(catechol) ones [137]. These findings suggest that PDA coatings grow progressively from the surface of the coated structure and the high cooperative adhesive action of these functional groups contribute to the resulting cohesive stable layer.
Table 2. Examples of polydopamine-coated nanosystems and the role PDA shell.
| Coated core | Preparation method of PDA layer | Properties/functionality of PDA layer | Application | Ref | |
|---|---|---|---|---|---|
| Inorganic cores | Gold nanostructures, like gold nanorods | Tris buffer (10 mM, pH 8.5)[DA] = 1 mg ml−1 Vortex and sonication for 35 min | Thickness = 30 nmPhotothermal effects Drug carrier for PDT (methylene blue) or CT (Doxorubicin) | PDT-PTT | [138] |
| Au–Ag branched NPs | Tris buffer (10 mM, pH 8.5)[DA] = 0.04–0.22 mg ml−1 3 h | Thickness = 6–34 nmPhotothermal effects Prevention of the release of toxic metals from the NPs core, enhancement of NPs biocompatibility Preservation of the branched structure of the NPs | Synergistic PTT | [139] | |
| Fe3O4 NPs | PBS buffer (10 mM, pH 8.5)[DA] = 0.12 mg ml−1 Shaking for 4 h | Thickness = 4 nmPhotothermal effects Adsorption of dye-labeled single-stranded DNA (ssDNA), for the detection of mRNA for cancer diagnosis Fluorescence quenching of the probe, hindering its off-target activation | MRI and PA imaging-guided PTT | [140] | |
| MnO2 NPs (mesoporous NPs) | Tris buffer (10 mM, pH 8.5)[DA] = 1 mg ml−1 6 h | Prevention of drug pre-leakage during circulation, pH-responsive drug release in the tumor acidic microenvironmentAnchoring of NH2-PEG-FA (for anti-fouling properties and active targeting of tumor) | PDT-PTT | [141] | |
| CaCO3 NPs | NH4HCO3 decomposed into CO2 and NH3 gas, ethanol, solution of Ca2+ and dopamineDopamine/CaCl2 = 2/150 (m/m) 24 h | Thickness = 44 nmPreparation of hollow PDA-CaCO3 nanoparticles Immobilization of a lipid bilayer of DOPA, DPPC, cholesterol and DSPE-PEG Loading of the PS Ce6 and prevention of its photobleaching pH-responsive drug release in the tumor acidic microenvironment | Imaging-guided PDT | [76] | |
| Silica NPs (mesoporous NPs) | Tris buffer (10 mM, pH 8.0)[DA] = 1.5 mg ml−1 2.5 h | Photothermal effectspH- and NIR-responsive drug release in the tumor microenvironment Anchoring of SH-PEG-FA (for anti-fouling properties and active targeting of tumor) | Targeted Chemo/Gene/PTT | [142] | |
| Organic cores | PLGA-TPGS NPs | Tris buffer (10 mM, pH 8.5)[DA] = 0.1 mg ml−1 6 h | Thickness ∼15 nm. Anchoring of SH-terminated aptamer (for active targeting of tumor and enhanced cellular uptake) | Targeted chemotherapy | [143] |
| Liposomes (POPC) | Tris buffer (10 mM, pH 8.5)[DA] = 0.2 mg ml−1 4 h, 40 °C | pH-responsive drug (5-FU) release in the tumor microenvironmentStable and biocompatible coating | Chemotherapy | [144] | |
| DSPE-PEG micelles | Tris buffer (10 mM, pH 8.5)[DA] 6 h | Thickness ∼4 nmPhotothermal effects Conjugation of chemotherapeutic drug (Bortezomib) pH- and NIR-responsive drug release in the tumor microenvironment | Chemo/photothermal therapy | [145] | |
| Polypeptides micelles (PEG45-b-poly(L-cysteine)20) | Tris buffer[DA] = 0.75 mg ml−1 4 h | Thickness ∼45 nmPhotothermal effects NIR-responsive drug (Doxorubicin) release in the tumor microenvironment | Chemo/photothermal therapy | [146] | |
| Soft cores | Emulsion droplets (DMDES) | Emulsion of DMDES, ammonia and SDSTris buffer [DA] = 1.4 mg ml−1 24 h Ethanol for DMDES removal | Photothermal effectsPreparation of PDA nanocapsules pH and NIR-responsive drug (doxorubicin) release in the tumor microenvironment | Imaging-guided chemo/photothermal therapy | [72] |
| Drug assemblies | Doxorubicin NPs (DNPs) | PBS buffer (pH 8.5)[DA] = 0.75 mg ml−1 Overnight | Photothermal effectsProlong blood circulation of DNPs and Prevent doxorubicin pre-leakage pH and NIR-responsive drug release in the tumor microenvironment | Chemo/photothermal therapy | [147] |
PLGA: poly(lactide-co-glycolide), TPGS: d-α-tocopheryl polyethylene glycol 1000 succinate, PEG: polyethylene glycol, 5-FU: 5-fluorouracile.
4.2.2. Metal binding ability
Catechol function is the major responsible of PDA affinity to several metal cations, including Fe2+, Fe3+, Cu2+, Mn2+, Pb2+ and multiple others. It can indeed strongly bind to multivalent metal cations through the o-diphenol functionality, and it exhibits a higher binding affinity compared to o-quinone [150]. This chemistry depends however on several conditions including the pH [54] or the affinity of catechol towards the metal ion. It should be noted that other functional groups present in PDA would also participate in metal chelating properties, such as amine, carboxylic and phenolic groups [30]. The PDA-metal coordination ability was explored for the preparation of diverse metal-organic frameworks (MOFs) [151–153] and has been considered of particular interest for the design of novel PDA-based theranostic systems. For instance, several magnetic resonance imaging (MRI)-guided nanoplatforms have been described in tumor imaging and were obtained through interaction of PDA with paramagnetic metal ions like Gd3+ [154], Fe3+ [54] or Mn2+ [155], leading to efficient MRI contrast nanoagents exhibiting high longitudinal relaxivity values in comparison to conventional contrast agents [30]. Such theranostic systems could be in fact achieved either by loading/anchoring the metal cations onto PDA nanostructures [54, 156] or via coating metal oxide cores (like Fe3O4 or Mn3O4) with a PDA shell [157, 158].
It was found that the chelation of metal ions would induce their reduction and subsequent transformation into metallic nanoparticles bound to PDA surface, this was used for the in situ growth of Au [159] or Ag [160] nanoparticles on PDA surface. In addition, the catechol reactivity towards metal cations has been largely explored in PDA synthesis process as described in section 1 and could be also used for the complexation of de-assembled PDA aggregates as a strategy to form cohesive robust PDA coatings [161]. Furthermore, it has been recently discovered that PDA nanoparticles can selectively kill cancer cells through a ferroptosis mechanism involving this metal complexation ability [162, 163]. In vivo, biological melanins are also well-known for their affinity towards metal ions [164], which is used for instance for the sequestration of potentially cytotoxic transition metals [9].
4.2.3. Chemical reactivity
The high chemical reactivity of PDA is particularly interesting for the design of multifunctional nanosystems and can serve to extend PDA nanomaterials applications.
4.2.3.1. Covalent interactions
4.2.3.1.1. Catechol/quinone reactivity ( figures 5(A) and (B))
The catechol group, a central element in PDA reactivity, is in fact present in a chemical equilibrium with its corresponding quinone [91], and the reduced/oxidized state of the catechol/o-quinone system is of great importance to direct PDA reactivity. This equilibrium can be impacted by the solution pH, the presence of oxidants/reductants, or by exposition to relatively high temperature under air.
O-quinone systems, owing to the carbonyl function and Michael acceptor character, are prone to addition reactions of nucleophilic groups such as thiols and amines, via Michael addition (for thiols and amines) or Schiff-base condensation (for amines). The most favorable positions for such addition reactions depend on the type of the engaged nucleophile and on the quinone system (dopamine-quinone or indole-quinone). For instance, Michael addition of thiols or amines occurs preferentially via 1,4-addition while carbonyl reactivity with amines is preferentially a 1,2-addition type yielding the Schiff-base [25]. This chemistry is one of the most explored strategies used for the immobilization of (bio)molecules, such as polymers [165, 166], proteins [167, 168] or small molecules [169], on the surface of PDA nanoparticles or shells (table 3). This is mainly attributed to its simplicity (since the reaction can be simply conducted under mild alkaline aerobic conditions) and to its versatility (since amines and thiols are present in numerous molecules or can be easily introduced into them) [25]. Poly(ethylene glycol) (PEG) chains grafting is for instance often achieved using this o-quinone reactivity, allowing the formation of a hydrophilic antifouling coating on the surface of PDA nanoparticles or PDA-coated nanosystems, permitting to improve their colloidal stability, reduce their uptake by the reticuloendothelial system and prolong their blood circulation times [45, 170, 171]. Also, tumor targeting molecules like FA [172], galactosamine [173] or EGFR antibody [174] could be grafted on PDA surface via this chemical route, in order to improve tumor accumulation for enhanced treatment efficiency and reduced side effects [30]. Few examples of the implementation of this chemistry are illustrated in table 3.
Table 3. Examples of Michael addition and/or Schiff base condensation reactions explored for the development of PDA-based nanosystems.
| Grafted molecule | PDA-based system | Reaction conditions | Applications | Ref |
|---|---|---|---|---|
| Thiolated poly(methacrylic acid) (SH-PMA)—pH cleavable hydrazine bond—Doxorubicin (DOX) | PDA NCs | pH 8.0Overnight incubation In presence of TCEP (to prevent the oxidation of thiol groups) | Immobilization of DOXPreparation of a stimuli-responsive DDS for an intracellular delivery of the anti-cancer drug | [165] |
| Thiol- or amine-terminated poly(ethylene glycol) | PDA NPs | ⩾pH 8.0Overnight incubations | Improvement of PDA NPs colloidal stability for in vivo applications | [170, 175] |
| Thiol-terminated hydroxyethyl starch (HES) | PDA NPs | pH 10.024 h incubation | Improvement of the PDA NPs colloidal stability for in vivo applications, using a coating with a favorable biocompatibility and biodegradability (superior to PEG-modified PDA NPs) | [166] |
| SH-PEG—folic acid (FA) | PDA-modified mesoporous silica NPs | pH 8.5In presence of TCEP (to prevent the oxidation of thiol groups) | Grafting of a cancer targeting moiety for enhanced anti-tumor therapy efficiency | [176] |
| FA | PDA-coated Au-Zein nanocomplexes | pH 8.51 h incubation | Grafting of a cancer targeting moiety for enhanced anti-tumor therapy efficiency | [169] |
| Galactosamine (amin-bearing tumor targeting moiety) | PDA NPs | pH 8.52 h incubation | Grafting of a tumor targeting moiety for specific liver tumor targeting | [173] |
| EGFR antibody | PDA-coated mesoporous silica nanoparticles | — | Grafting of a tumor targeting moiety for specific tumor targeting | [174] |
| Ovalbumin (amin-bearing tumor model antigen) | PDA NPs | Neutral conditions5 h incubation | Grafting of a tumor antigen on PDA NPs for antigen delivery in tumor immunotherapy | [167] |
| Bovine serum albumin (BSA) | Graphene oxide/PDA hybrid nanosystem | pH 8.524 h incubation | Immobilization of BSA, serving as a molecular carrier for paramagnetic agents | [168] |
| Arginine | PDA NPs | Tris buffer72 h incubation | Attachment and packing of in situ formed PDA nanoparticles on the surface of linoleic acid-arginine nanoemulsion droplets, used as templates for the preparation of PDA nanocapsules | [177] |
| Polyethyleneimine (PEI) | In situ formed polydopamine nanoparticles | — | Co-polymerization of dopamine and PEI for the preparation of fluorescent polydopamine nanoparticles, through the reduction of π–π interactions | [178] |
NPs: nanoparticles, NCs: nanocapsules
4.2.3.1.2. Amine reactivity (figure 5(C))
In addition to the catechol/o-quinone system, the amine groups also contribute to the chemical reactivity of PDA through interaction with electrophilic moieties or exhibiting an acid/base reactivity [25]. For instance, amine functions present in PDA can interact with carboxylic groups to form an amide function via conventional carbodiimide chemistry reported as a drug coupling strategy [179], or serve as a reactive site for facile coupling of isothiocyanate-containing molecules that can be particularly used for PDA nanoparticles labeling. Moreover, an amine-mediated acylation reaction was employed to immobilize an ATRP initiator on PDA-coated particles allowing for a surface-initiated polymerization and controlled growth of polymer brushes from the particles surface [180]. Amines are also involved in aza-Michael-type addition which was used for PDA functionalization using acrylate/acrylamide molecules [181].
4.2.3.2. Non-covalent interactions (figures 5(B) and (D))
Due to the presence of catechol groups and pyrrolcarboxylic acids (acidic) on one hand and amines groups (basic) on the other hand, PDA displays a zwitterionic character with an isoelectric point of ∼4 [45, 182]. PDA can hence expose positive or negative charges depending on the medium's pH. This functionality was explored for the highly selective pH-responsive uptake and release of charged small molecules into PDA capsules [182], and represented an interesting approach for controlled drug loading and release in the drug delivery applications.
Beside electrostatic interactions, PDA assemblies are able to interact with diverse molecules through other non-covalent interactions, namely π–π stacking, hydrophobic effects and van der Waals interactions and H-bonding. Indeed, the abundance of π-conjugated, aromatic and phenolic structures in PDA network allowed for multiple drugs, including doxorubicin [64, 183] and paclitaxel [184] or photosensitizing molecules like chlorin e6 [185] and indocyanine green (ICG) [171, 183], to be loaded in the PDA matrix [64, 185] or bound onto PDA surface [183], affording thus PDA-based therapeutic platforms working as promising candidates for efficient cancer treatment.
4.3. UV-visible-NIR absorption properties
Unlike common organic products, synthetic eumelanin-like PDA displays a broad-band absorption extending over the whole UV-visible-NIR regions, and increasing monotonically towards high energies [9]. This unique profile has been reported before as a characteristic optical feature for natural melanins (eu-, neuro-, allo- and pheo-melanins) [186–188]. The extinction coefficients calculated for the wavelengths ranging from 300 to 1000 nm for both PDA nanoparticles and melanin granules were found to be very close, especially for the smallest nanoparticles [45]. Based on these spectral similarities, analogies were made between the two resembling materials for a better interpretation of PDA optical properties.
For eumelanin, contrary to what has been though at first, the broad-band absorption does not originate from scattering phenomena [189] but corresponds effectively to an intrinsic light absorption. Indeed, based on accurate measurements of the optical scattering coefficient, it was found that the contribution of Rayleigh scattering is lower than 6% of the total optical attenuation in the 210–325 nm region and is undetectable for the wavelengths ranging between 325 and 800 nm [187]. Similarly, Warren et al confirmed recently that, for PDA also, the optical spectrum reflects real absorption and is not significantly altered by scattering effects [129].
The 'chemical disorder model' is accordingly one well-accepted explanation of this broad absorption band characterizing eumelanin and synthetic eumelanin-like materials, particularly PDA [188, 190]. In this model, the monotonic absorption profile is the consequence of the overlapping of different absorption bands which can be attributed to ensembles of chemically distinct species present in the material network [188].
Figure 6. Simplified scheme of chemical disorder levels in eumelanins realized through structural definitions at multiple length-scales in the hierarchy of the material (Adapted from [122]).
Download figure:
Standard image High-resolution imageAlthough the chemical disorder model can be used for the understanding of the broad absorption, the structural complexity at the origin of such absorption can not only be related to the chemical heterogeneity at the level of fundamental building units but also to the hierarchical assembly configurations (at secondary/supramolecular level), which produce a wide series of highest occupied molecular orbital (HOMO)—lowest unoccupied molecular orbital (LUMO) gap energies, resulting in the monotonic featureless broad absorption band [26, 190]. In this context, few models have been suggested to interpret the unique absorption profile of natural or synthetic eumelanins (Figure 6). Indeed, the oxidation/reduction dynamics among eumelanin-like oligomers and the aggregation-dependent interchromophoric interactions were presented as important factors perturbing π-electron delocalization [191, 192], resulting in different HOMO-LUMO gaps. The oxidized forms were admitted to have lower HOMO-LUMO gap energy, thus leading to a broadening of eumelanin absorption band.
More recently, the geometric order/disorder model emerged as another key factor to consider in the interpretation of the broad absorption of eumelanin and PDA. The significant contribution of non-covalent interactions in the assembly process of PDA, including π–π stacking, π-cation interactions and charge transfer, has been well-established using experimental and computational structural investigations. Since π-electron density of eumelanin-like oligomers is an important parameter impacting their absorption profiles, the proximity between oligomers but also the packing between stacked oligomers would impact the absorption spectrum of the assembled structure [129, 192–194]. Chen et al showed that the excitonic effects of the eumelanin aggregated stacked oligomers (protomolecules) has a great role in the broad-band absorption. The protomolecules being in different sizes and randomly oriented, the interplay of the geometric order/disorder in the aggregated eumelanin structures would result in random and significant excitonic couplings through the formation of electron-photon pairs, leading to the broadening of the absorption chromophore band along with the enhancement of the absorption towards higher energies [193]. The contribution of aggregation and structural scaffold disorder, as well as the redox dynamics, in the eumelanin chromophore broad band were also investigated and confirmed by Micillo et al [194].
These findings were reinforced very recently by Warren's group [129] who conducted an in-depth investigation of the optical properties of eumelanin-like nanoparticles, originating from dopamine or DOPA, by means of pump-probe microscopy technique, which allowed to reveal features of individual absorption bands. Considering that PDA nanoparticles originate from a multi-step process consisting in (a) the formation of oligomeric building blocks, (b) their consecutive stacking followed by (c) the aggregation of these stacked oligomers, the authors examined the absorption features at these different assembly levels (figure 7). It was found here that LMW subunits present higher UV absorption compared to larger fragments, while HMW fractions showed an enhanced absorption at the visible and NIR wavelengths compared to LMW units. A possible explanation suggested that HWM fractions would be composed of higher MW oligomers, leading to more extended π-electron delocalization within oligomers and thus to a lower HOMO-LUMO gap energy. A higher stacking order between oligomers of different HOMO-LUMO gaps would enhance the absorption at low energies [129, 195]. Interestingly, the authors revealed that HMW fragments exhibited different absorption behavior compared to their parental nanoparticles. This implied that a change in the electronic structure would occur when the stacked oligomers aggregated to form the nanoparticle. A size-dependent absorption was also found for PDA nanoparticles; larger nanoparticles displayed higher absorption at longer wavelengths compared to smaller ones. Based on these observations, Warren's group concluded that, in addition to the chemical diversity model and the fundamental oligomers' π-electron delocalization, the geometric packing order of the protomolecules in eumelanin-like systems is a key determinant in the broad-band absorption of the final aggregated assembly; it may impact the interactions between oligomers changing thus the absorption behavior. The number of stacked oligomers would impact the geometric preference, which results in the size-dependent absorption profiles. The higher absorption observed at longer wavelengths for large nanoparticles in comparison to smaller ones was also reported by Wang et al [92]. It should be noted, that the geometric packing order model detailed by Warren's group is well-adapted to interpret the size-dependent properties of PDA nanoparticles, namely the nanomechanical [52] and photothermal properties [92], as will be discussed later.
Figure 7. Structure-property relationship proposed by Warren's group for melanin-like materials including polydopamine, based on the geometric disorder model. The hierarchical assembly would play a role to extend the broad absorption band to low energies (adapted with permission from [129]). Reprinted with permission from [129]. Copyright (2018) American Chemical Society.
Download figure:
Standard image High-resolution image4.4. Fluorescence emission properties
An investigation of the absolute radiative quantum yields of synthetic eumelanin was performed by Meredith et al and showed that more than 99.9% of the absorbed light in the UV and visible regions was dissipated through non-radiative relaxation mode [196], which concurred with previously reported findings revealing an extremely low emission quantum yield (Φr as low as 0.003) [197, 198]. Comparably to other synthetic eumelanins, very weak emission spectra were reported for conventionally-prepared PDA nanoparticles [199]. Similar to self-assembled organic chromophore systems, the stacking of fundamental oligomers and the subsequent aggregation in PDA nanomaterials were proposed to be at the origin of the observed self-quenching effects [199].
Interestingly, although very weak, the emission of synthetic eumelanins showed a clear dependency of the position, width, intensity and quantum yield on the excitation energy, which is an uncommon feature for organic materials. Lower excitation energies led to lower radiative emission, with for example a four-fold increase of the quantum yield at a 250 nm excitation compared to 500 nm [196, 200]. A 3D map of specific quantum yields variation as a function of excitation and emission wavelengths was developed to help understand the photophysical and structural properties of eumelanin-like materials [200]. The photoluminescence was proposed to arise from different HOMO-LUMO gap energies related to oligomers of chemically distinct species, supporting hence the chemical disorder model described above [196]. This hypothesis was supported in other reports which related eumelanins emission properties to the chemically distinct oligomeric units that can be selectively excited at different excitation wavelengths [200, 201]. These interpretations can be also applied to PDA [26].
Furthermore, size-dependent emission spectra and yields were obtained for eumelanin samples [197]. The small eumelanin aggregates exhibited higher emission yields with longer emission decay times (>1 ns for <1000 Da) compared to larger ones (<1 ns for >10 000 Da). Also, it was found that energy transfer occurs both within but also between the fundamental building units. Thus, the aggregation state, responsible for eumelanin-like materials buildup, was proposed as another determinant factor in this photophysical property [197, 198].
Interestingly, several groups have shown that fluorescent PDA nanomaterials can be made by tailoring PDA synthesis or post-processing conditions. Endowing PDA products with luminescence properties can be indeed of great utility for the development of nanosystems used in medical imaging or fluorescence bioimaging-guided therapies. Different fabrication strategies have been developed in order to achieve the desirable photoluminescence properties of PDA and were categorized, by Yang et al [202], into four strategies consisting in PDA (a) chemical oxidation [203], (b) degradation [204], (c) conjugation [178] or (d) carbonization [205]. These strategies were mainly based on the reduction/weakening of the π–π interactions present in PDA scaffold or on the reduction of dopamine polymerization degree, hindering hence the aggregation-caused quenching effect, or also on the creation of distinct sp2 and sp3 hybridized carbon atoms, introducing thus the fluorescence properties.
For instance, the oxidation of PDA particles using hydrogen peroxide has emerged as a facile novel method allowing the synthesis of fluorescent organic nanoparticles with a tunable fluorescence emission [206–208]. Interestingly, the obtained PDA nanoparticles displayed two-photon fluorescence properties under NIR light excitation, which permits to reach a superior penetration depth during tissue bio-imaging. The same route was also reported for the preparation of fluorescent PDA shells and the development of fluorescent PDA nanocapsules [35]. Besides, based on the high ROS rates, and particularly H2O2, in the tumor microenvironment in comparison to normal tissues, PDA oxidation-mediated fluorescence generation can be explored for tumor diagnosis/operation applications [207].
4.5. Photothermal and photoacoustic properties
The conversion of light into heat by a photosensitizing agent is called photothermal effect. There are different mechanisms for light conversion into heat that depend on the nature and the structure of the photothermal agents. These mechanisms can be classified into three main categories (figure 8):
Figure 8. A simplified representation of the different photothermal conversion mechanisms.
Download figure:
Standard image High-resolution image- Plasmonic heating which is observed with some plasmonic metal nanoparticles such as silver, copper and gold [209, 210]. In these materials, the metal atoms are surrounded by delocalized electrons that can move freely within the metal. A plasmon resonance phenomenon which is a collective oscillation of electrons occurs when the wavelength of the incident light matches the natural frequency of electrons on the surface of metals. This coherent oscillation of electrons with the incident electromagnetic field results in the generation of heat.
- Electron–hole generation and relaxation which is the common mechanism of heat conversion for semiconductors materials that generate above-bandgap electron–hole pairs upon the absorption of light energy higher than the bandgap. These above-bandgap electron–hole pairs will then relax to the band edges with subsequent conversion of the extra energy into heat [211].
- Fluorescence quenching and vibrational relaxation observed in supramolecular assemblies of pigments. Indeed, because of the densely packed pigments organization inside the assemblies, their fluorescence is completely quenched, thus, upon their illumination at a specific wavelength, the absorbed photonic energy usually released as fluorescence and singlet oxygen is dissipated thermally through vibrational relaxation [212, 213].
PDA products are able to generate heat through non-radiative relaxation when they are photo-excited due to their intrinsic fluorescence self-quenching effect [26]. Indeed, a transient pump-probe analysis performed on eumelanin-like samples showed a very rapid ground-state bleaching with about 90% of the absorbed energy dissipated as heat, within a relaxation time of 20 ps [198].
This characteristic feature, together with the broad light absorption band, paved the way for the eumelanin-like PDA nanomaterials to be investigated as potential agents used in hyperthermia-mediated therapy called PTT. This therapeutic approach is based on the administration of a light-heat converting agent followed by the illumination of the targeted site with a localized laser. The heat generated locally by the photothermal agent, upon its photo-excitation, is used to kill abnormal cells nearby. PTT has been largely explored in the past years as a minimally invasive therapeutic method showing promising results in efficient tumor ablation, but also in other applications such as multi-resistant bacterial infections treatment. This method is recognized as a particularly advantageous treatment modality, compared to the conventional cancer therapies owing notably to the spatio-temporal controllability which allows improved target selectivity and minimal side effects, with subsequent efficiency enhancement [30]. The photothermal agents currently available are mainly consisting in metallic nanoparticles, particularly Au nanoparticles, and few carbon-based nanosystems. However, these nanoagents usually suffer from long-term biosafety, compromising their clinical implementation [46]. The application of biocompatible and biodegradable materials in photothermal therapies is hence of great interest for the in vivo biomedical applications.
Liu et al have introduced, for the very first time, melanin-like PDA nanoparticles as a powerful photothermal agent fulfilling all the requirements for an in vivo PTT application [46]. Colloidal PDA nanospheres displayed a strong NIR photo-absorption, with a molar extinction coefficient of  808 nm = 7.3 108 M−1 cm−1 (for a nanoparticles size of 70 nm), which is relatively high in comparison to values reported for multiple photothermal agents such as carbon nanotubes (
808 nm = 7.3 108 M−1 cm−1 (for a nanoparticles size of 70 nm), which is relatively high in comparison to values reported for multiple photothermal agents such as carbon nanotubes ( 808 nm = 7.9 106 M−1 cm−1) [46]. A strong absorption in the so-referred-to 'photo-therapeutic window' (650 nm–850 nm) is in fact highly interesting for clinically relevant photothermal applications. In this optical window, there is a minimal interference of biological chromophores absorption or scattering effects and a maximal penetration depth of the incident light is enabled [31, 214].
808 nm = 7.9 106 M−1 cm−1) [46]. A strong absorption in the so-referred-to 'photo-therapeutic window' (650 nm–850 nm) is in fact highly interesting for clinically relevant photothermal applications. In this optical window, there is a minimal interference of biological chromophores absorption or scattering effects and a maximal penetration depth of the incident light is enabled [31, 214].
The photothermal conversion efficiency is another key parameter indicative of the photothermal agents' efficiency. In the case of PDA nanospheres of 70 nm, the photothermal conversion efficiency was found to be about 40% [46]. This value is interestingly high compared to the most common PTT agents, such as gold nanorods, widely reported for cancer treatment, which exhibit a photothermal conversion efficiency of 22% [46]. Indeed, upon 500 s of illumination with an 808 nm laser at 2 W cm−2, these PDA nanospheres dispersed at 200 µg ml−1 induced a temperature increase of the suspension of almost 34 °C. This result is very satisfying for high-temperature hyperthermia-mediated tumor ablation, since it is accepted that cancer cells, which are more sensitive to hyperthermia than normal cells [215], can be killed when the surrounding temperature increases to values >50 °C for 4–6 min, or 42 °C–45 °C for 15–60 min [216].
These findings were later confirmed by several groups who reported a temperature increase of a similar order of magnitude. The generated temperature increased in a concentration- and laser power-dependent manner [54, 64, 179, 217, 218]. Similar to the colloidal nanospheres, multiple PDA-based nanostructures, such as PDA shells [219–221], PDA dots [222] and mesoporous PDA nanostructures [223], showed excellent photothermal properties relevant to PDA nanostructure. In the case of core@PDA shell systems, an improved photothermal effect could be obtained by increasing PDA shell thickness [219, 220].
Although the photothermal properties of PDA materials have been demonstrated by several groups, a full understanding of this photothermal conversion ability is still lacking. Particularly, to the best of our knowledge, no work has yet studied in depth the impact of PDA nanoparticles size on their photothermal conversion efficiency. In one recent study, Wang et al have briefly revealed a dependency of the solution temperature increase on the size of PDA nanoparticles; larger nanoparticles induced higher temperature increase compared to smaller ones. This result was attributed here to the higher absorption in the NIR region (808 nm) observed for larger nanoparticles [92]. A similar result was also reported for mesoporous PDA nanoparticles [223]. These findings could be interpreted by the geometric packing order/disorder model and the aggregation state of the stacked oligomers forming PDA nanoparticles, as explained before: bigger PDA particles would exhibit a higher geometric packing order [129], and thus higher excitonic effects, NIR absorption and quenching effects, leading to the stronger heat generation.
Interestingly, when PDA nanoparticles were subjected to a long-time laser illumination (2 W cm−2 for 1 h) [46, 54] or repeated illumination-cooling cycles (up to five cycles) [175, 217] they showed a high photo-stability as evidenced by the stable temperature increase obtained at the different cycles, by the stable absorption features and by the absence of morphological changes before and after illumination. Such property is crucial for the maintenance of efficient light absorption during NIR irradiation, and renders PDA-based nanostructures superior to gold nanoparticles, largely reported in PTT applications, which undergo morphological changes resulting in decreased light absorption during NIR activation [46].
The excellent photothermal stability during cyclic irradiation, along with the strong light absorption and great photothermal activity and conversion efficiency, suggested that PDA nanoparticles would be of a great potential in photoacoustic imaging (PAI) applications. This technique is in fact an emerging bio-imaging method allowing to dynamically monitor the biodistribution and tumor retention of injected systems and offering a high tissue penetration depth, spatial resolution and sensitivity. It relies on pulsed illumination of a photo-thermal converting agent, the heat generated by this latter would thermo-elastically generate pressure waves detected using an ultrasound transducer [30, 224]. The combination of photoacoustic and photothermal effects in a single system would be highly interesting for PAI-guided cancer PTT applications, offering hence a desirable theranostic platform [225]. Aqueous PDA nanoparticles suspensions exhibited a concentration-dependent photoacoustic performance and interestingly photoacoustic signals could be detected even at concentrations as low as 15 µg ml−1. In vivo assays revealed the excellent photoacoustic contrast generated by PDA nanoparticles and tumor-targeted PDA nanoparticles enabled significant illumination of the tumor site with a clear delineation of margins. Several other studies followed and confirmed the great potential of PDA-based nanosystems for PAI applications [223, 226–228]. PDA-coated nanostructures were also proven to exhibit excellent photoacoustic properties. For instance, 200 nm sized PDA hollow nanocapsules dispersed at 200 µg ml−1 exhibited a slightly higher photothermal conversion efficiency (40.4% versus 37.1%) and over two folders higher PAI ability in comparison to PDA nanospheres of similar size and at similar concentration [72]. The higher photoacoustic signals were attributed to the higher photothermal conversion ability, but also to the hollow structure favorable for harmonic imaging enforcing the ultrasonic signals.
5. Application of PDA nanomaterials in cancer therapy
5.1. Toxicity, biocompatibity and biodegradability
Based on structural and physico-chemical similarities reported between synthetic eumelanin-like PDA and naturally occurring melanin, PDA materials were anticipated to benefit from the wide distribution of melanin in the human body and show excellent biocompatibility [26, 46]. Numerous in vitro and in vivo studies performed on PDA-based nanosystems (PDA nanospheres, PDA nanocapsules, PDA-coated nanoparticles) confirmed their biocompatibility and revealed their great potential as candidates for in vivo biomedical applications. However, the presence of dopamine monomers in PDA matrix, as revealed in some reports [115], have raised questions regarding the possible cytotoxic effects that could be related to this neurotransmitter. To address this issue, Hong et al examined the amount of dopamine released from PDA particles. Their findings revealed an extremely low-level release of dopamine, which was attributed to a robust self-assembled structure in which dopamine is well-entrapped. Cytotoxicity assays showed a viability maintained to >90% with preserved growth and morphology [115], which was consistent with the excellent biocompatibility of PDA widely documented in the literature. Indeed, PDA-based nanosystems showed negligible toxicity on a wide set of cells tested in vitro (epithelial cells, endothelial cells, fibroblasts, osteoblasts, neurons, etc), for which viability and proliferation ability were almost maintained to normal [46, 162, 163]. In agreement with these in vitro assays, in vivo studies confirmed the excellent biocompatibility of PDA-based nanosystems used in various contexts [158, 228]. For instance, PDA nanoparticles injected intravenously in rats showed a very low acute toxicity with a very high median lethal dose (about 500 mg kg−1), nearly five hundred times the dose used for their photothermal therapeutic applications. Interestingly, long-term in vivo toxicity monitored over a 1 month period after a single intravenous injection in mice or rats revealed that the animals remained healthy with no sign of behavior abnormalities; complete blood panel tests showed normal biochemical indicators with no detectable interference with the physiological regulation or immune response. Moreover, the histopathological examination did not show any sign of tissue damage, inflammatory, or fibrosis aspect with no detectable change in the cellular structures [46, 54]. These findings were in line with the results reported for melanin showing no noticeable toxicity detected within 30 d, after injection of high doses (up to 100 mg kg−1) in mice [229]. Furthermore, multiple evidences indicated that PDA coatings, shown to be ultra-stable in vivo, can interestingly function as a biocompatible layer allowing to significantly attenuate the intrinsic toxicity of biomaterials in a material-independent manner. Indeed, several reports have highlighted the beneficial role of PDA coatings in reducing the inflammatory and immunological side responses and improving the biocompatibility of various materials including organic materials (like poly-L-lactic acid), metallic nanoparticles (like gold nanoparticles), quantum dots, etc [51, 230–232]. PDA coatings also showed a beneficial role in promoting hemocompatibility and controlling cell adhesion and proliferation on substrates [233, 234]. Increasing the hydrophilicity of the tissue-contacting interfaces was proposed as an important factor responsible for the reduction of the in vivo side effects in PDA-coated biomaterials in contact of tissues or blood.
Beside their high biocompatibility, several findings reported in the literature led to the identification of PDA as a biodegradable material. Its naturally occurring analogue, i.e. eumelanin, is assumed to be metabolized in vivo, although the exact biodegradation pathways are not fully known yet. Langer's group revealed that synthetic melanin implants could be significantly resorbed within 8 weeks, which was attributed here to an uptake of melanin particles by macrophages and giant cells and to a further intracellular degradation process [235]. In fact, it has been demonstrated that both PDA and natural melanin undergo a hydrogen peroxide-mediated decomposition related to the oxidative cleavage of o-quinone-indole moieties leading to the formation of pyrrole-2,3-dicarboxilic acids (PDCA) or pyrrole-2,3,5-tricarboxylic acids (PTCA), depending on branching positions in covalent oligomers [42, 46]. This observation is highly interesting for the understanding of the possible biodegradation process of PDA, since hydrogen peroxide and other reactive oxygen species (ROS) are endogenous components that can be produced majorly by NADPH oxidases, which are enzymes largely distributed in phagocytes and many organs [26, 46, 236]. This oxidation-induced process is generally accepted as a likely degradation pathway of PDA materials in vivo.
These intrinsic biocompatibility and biodegradability properties of PDA materials make them highly suitable for in vivo applications.
5.2. Drug delivery applications
The administration of free anti-cancer drugs usually encounters several limitations, including particularly solubility issues or a non-specific biodistribution leading to a poor drug bioavailability and severe side effects [30]. In order to overcome these limitations, diverse drug delivery nanosystems have been developed with several successful clinical implementations, benefiting from their passive tumor accumulation via the EPR effect [237]. In this context, owing to its high chemical reactivity resulting in high drug payloads and its multi-drug loading ability, PDA-based nanostructures have received considerable attention as drug carriers allowing drug/gene delivery for efficient cancer treatment. Moreover, when the anti-cancer drug is physically loaded on the nanocarrier, the H-bonding and π–π interactions generally established between PDA and the loaded drugs tend to be disrupted or weakened under acidic, oxidative or also reductive conditions. Therefore, PDA nanostructures would benefit from the intrinsic tumor microenvironment characteristics (i.e. weakly acidic pH, high amounts of hydrogen peroxide and of glutathione) and function as stimuli-responsive drug delivery systems [30]. Moreover, taking advantage of the endocytosis-mediated internalization process of PDA nanoparticles demonstrated by Ding et al [238], drug release can be triggered in the acidic endosomal and lysosomal compartments allowing the intracellular delivery of the anti-cancer drug. Additionally, the hyperthermia generated by PDA upon its NIR illumination could also be explored to achieve an on-demand NIR-triggered drug delivery, as a result of weakened interactions with the loaded drug under heat [239].
In view of such features, PDA-based nanomaterials have been largely explored as drug nanocarriers, few examples are cited in table 4. They showed excellent results in preventing the premature drug release during circulation and inducing enhanced release rates after tumor internalization, compared to pristine or other nanoparticles. PDA nanocapsules and mesoporous PDA nanoparticles are particularly promising candidates for drug delivery applications, owing to their high payload capacity [177]. Regarding the PDA-coated structures, PDA coatings can serve either as the drug nanocarrier composite [240], controlling the thickness of PDA shell allows here to control the drug loading rate, or serve as a 'gate keeper' for a controlled drug delivery, owing to its stimuli-responsiveness [241].
Table 4. Examples of PDA-based nanoplatforms used for mono- or multi-modal cancer therapy.
| Nanosystem configuration | Targeting moiety | Cargo | Applications | Properties | Testing stages | Ref |
|---|---|---|---|---|---|---|
| PDA NPs | Ovalbumin (tumor model antigen) | — | Immunotherapy | Vaccine delivery | In vitro: C57BL/6 (murine colon cancer)In vivo: colon-cancer model | [167] |
| Fe3O4-PDA NPs | Bortezomib (BTZ) | — | CT,MRI guidance | pH-responsive drug release:
| — | [242] |
| Magnetic Nanocrystal Clusters (MNCs) @ PDA shell | Cisplatin | — | CTMagnetic guidance | pH-responsive drug release:
| In vitro: HeLa cells (human cervical cancer), MCF-7 cells (human breast adenocarcinoma) | [240] |
| Mn/SS/PEG-PDA NPs | Doxorubicin (DOX) | — | CTMRI guidance | pH and redox-responsive DOX release
| In vitro: 4T1 cells (breast cancer), HeLa cells, K7M2 (murine osteosarcoma)In vivo: 4T1-bearing tumor mice (breast cancer model) | [243] |
| PDA-modified MSNs | DOX | FA | Targeted CT | pH-responsive DOX release:
| In vitro: HeLa cellsIn vivo: HeLa-bearing tumor mice (cervical cancer model) | [176] |
| PEG-PDA NPs | Doxorubicin (DOX) | Triphenyl-phosphonium | Targeted CT | Mitochondria-targeted drug delivery for overcoming cancer drug resistance | In vitro: MDA-MB-231 cells (human breast adenocarcinoma) | [244] |
| PDA-coated CPT nanocrystals | Camptothecin (CPT) | Peptide XQ1 | Targeted CT | Improved solution dispersion and stability of CPT nanocrystalsRapid cross-membrane translocation and high intracellular drug delivery pH-dependent drug release | In vitro: A549 cells (human adenocarcinoma lung), HeLa cells | [245] |
| PDA-PEG NPs | DOX7-ethyl-10-hydroxycamptothecin (SN38) | — | CT, PTT | pH-responsive drug release:
*DOX and SN38, respectively:
| In vitro: MCF-7 cells, PC-9 (non-small-cell lung cancer) In vivo: PC-9 cells-bearing mice | [239] |
NIR irradiation-responsive drug release:*DOX and SN38, respectively:
| ||||||
| H2O2-responsive drug release |
CT: chemotherapy, NPs: nanoparticles.
PDA-based nanomaterials were in fact not only applied for the delivery of chemotherapeutic drugs, but were also extended to antigen delivery for anti-tumor immunotherapy [167]. They have emerged also as nanocarriers for photosensitizing agents used in PDT.
5.3. Light-mediated cancer therapies
Considering their outstanding photophysical properties, their colloidal stability and their superior biocompatibility and biodegradability, PDA-based nanosystems are emerging as attractive photothermal converting and photoacoustic-contrast agents and gaining an increasingly growing interest for efficient tumor ablation in PTT-based cancer therapy. Coupling PDA nanoparticles to photoactive molecules also known as photosensitizers, would present several advantages for cancer therapy. Indeed, PDA nanoparticles may play the role of nanocarriers of the photosensitizers with a high payload. Moreover, this enables the design of bifunctional light activated nanoplatforms with subsequent photothermal effect (PTT) and photodynamic effect (PDT). Such combination would allow a more efficient and synergistic treatment modality of cancer. In the following section, we will first point out the interest of PDA based materials as photothermal agents for cancer therapy, their implementation as nanocarriers of photosensitizers for PDT applications, and finally we will develop the various strategies that can be adopted for the design of PDA based nanoparticles with bifunctional PTT/PDT applications. In addition, the interests and limits of the bifunctional nanoplatforms in cancer therapy will be also emphasized and discussed. Few examples of PDA-based nanosystems applied in PTT and/or PDT cancer treatment are illustrated in table 5 .
Table 5. PDA-based nanoplatforms applied in light-mediated cancer therapy, PDT and/or PTT.
| Nanosystem configuration | Targeting moiety | Loaded PS | Properties | Applications | Illumination conditions | Testing stages | Ref |
|---|---|---|---|---|---|---|---|
| Ce6-PDA NPs | — | Ce6 | Enhanced cellular uptake of Ce6 | PTT/PDT: Increased cell killing effect of PTT/PDT combination in comparison to single treatment modality | PTT/PDT: 665 nm, 250 mW cm−2, 15 min | In vitro: T24 cells (bladder cancer) | [185] |
| Ce6-PDA NPs | — | Ce6 | Enhanced cellular uptake of Ce6 | PTT/PDT: Increased cell killing effect and in vivo therapeutic efficacy of PTT/PDT combination in comparison to single treatment modality | PTT: 808 nm, 2 W cm−2, 5 min for in vitro and 10 min for in vivo tests; | In vitro: HepG2 cells (liver cancer) | [179] |
| PDT: 670 nm, 50 mW cm−2, 5 min for in vitro and 10 min for in vivo tests | In vivo: HepG2 -bearing mice | ||||||
| Ce6-PDA-HA NPs | Hyaluronic acid (HA) | Ce6 | Tumor targeting and enhanced cellular internalization (CD44-mediated endocytosis) | Targeted PTT/PDT: Synergistic cell killing effect and enhanced in vivo therapeutic efficacy in comparison to single treatment modality | PTT: 808 nm, 2.5 W cm−2, 5 min for in vitro and 10 min for in vivo tests; | In vitro: HCT-116 cells and 3D tumor spheroids (colorectal cancer) | [246] |
| PDT: 670 nm, 50 mW cm−2, 5 min (for in vitro tests) or 10 min (for in vivo tests) | In vivo: HCT116 -bearing mice | ||||||
| PDA-Ce6-GSH-Au nanoflowers (NFs) | — | Ce6 (conjugated to the Au via GSH) | PDA coating resulted in a significant red shift of ∼80 nm and amplified the photothermal conversion efficiency of AuNFs | PTT/PDT: Enhanced photothermal conversion efficiency resulting from the association of PDA and Au NFs, Synergistic therapeutic effects of PTT/PDT combination in vitro and in vivo in comparison to single treatment modality.PDT: 660 nm, 100 mW cm−2, 5 min (for in vitro tests) or 10 min (for in vivo tests) | PTT: 808 nm, 2 W cm−2, 10 min (for in vitro and in vivo tests); | In vitro: HeLa cells (cervical cancer)In vivo: HeLa -bearing mice | [247] |
| Ce6-TPP-PDA@Black phosphorus (BP) nanosheets (NSs) | Triphenyl phosphonium (TPP) | Ce6 | Mitochondria-targeting systemFluorescence and thermal-guided imaging | Mitochondria-targeted PTT/PDT: Enhanced photothermal conversion efficiency resulting from the association of PDA and BP NSs, Synergistic therapeutic effects of PTT/PDT combination in vitro and in vivo in comparison to single treatment modality | PTT/PDT: 660 nm, 0.5 W cm−2, 5 min (for in vitro tests) or 10 min (for in vivo tests). | In vitro: HeLa cellsIn vivo: HeLa-bearing mice | [248] |
| ZnO-Ce6@PDA | — | Ce6 | Enhanced cellular uptake of Ce6 | PTT/PDT: Synergistic cell killing effect of PTT/PDT combination in comparison to single treatment modality | PTT: 780 nm, 2.9 W cm−2, 10 min (for in vitro tests);PDT: 660 nm, 100 mW cm−2, 10 min (for in vitro tests) | In vitro: HeLa cells | [249] |
| ZnP@PDA NPs | — | ZnP (Zinc Porphyrin) | Fluorescence-guided imagingFRET effect resulting in quenched ZnP fluorescence and improved PDA photothermal performance. | PTT/PDT: Enhanced photothermal conversion efficiency resulting from the association of PDA and ZnP, Synergistic therapeutic effects of PTT/PDT combination in vitro and in vivo in comparison to single treatment modality. | PTT/PDT: 660 nm, 0.8 W cm−2 for 5 min (for in vitro tests) or 0.75 W cm−2 for 5 min (for in vivo tests).In vivo: HeLa-bearing mice | In vitro: HeLa cells | [250] |
| C60-FA-PDA-GO nnaohybrids | FA | C60 (fullerene) | Tumor targetingReduction of GO due to PDA redox activity, which results in improved NIR absorbance and PTT performance | Targeted PTT/PDT: Enhanced photothermal conversion efficiency resulting from the association of PDA and reduced GO, Synergistic cell killing effect of PTT/PDT combination in comparison to single treatment modality | PTT/PDT: Xe lamp, 660 nm, 2 W cm−2, 9 min (for in vitro tests). | In vitro: HeLa cells | [251] |
| Ce6-MnO2-PDA-FA NPs | FA | Ce6 | Tumor targetingpH-responsive drug release, O2-generating system owing to MnO2 | Targeted and O2-strengthened PDT/PTT: cumulative photothermal effect resulting from the association of PDA and MnO2, Enhanced therapeutic efficacy of PTT/PDT combination in vitro and in vivo in comparison to single treatment modality | PTT: 808 nm, 1 W cm−2, 10 min (for in vitro and in vivo tests);PDT: 660 nm, 400 mW cm−2, 5 min (for in vitro and in vivo tests) | in vitro: MCF-7 cells (Breast cancer)in vivo: MCF-7-bearing mice | [141] |
| AIEgen-PDA-PEG NPs | Indolium group | AIEgens (TPE-Methoxyl-indolium) | Mitochondria-targeting,Fluorescence-guided imaging | Imaging-guided PDT-PTT: Synergistic therapeutic effects of PTT/PDT combination in vitro and in vivo in comparison to single treatment modality | PTT: 808 nm, 1 W cm−2, 5 min (for in vitro and in vivo tests);PDT: white light, 100 mW cm−2, 10 min (for in vitro and in vivo tests) | In vitro: HeLa cellsIn vivo: HeLa-bearing mice | [252] |
| ICG-Laponite-PDA-PEG-RGD NPs | Arginine-Glycine-Aspartic acid (RGD) | Indocyanine green (ICG) | Improved photostability of ICG in the presence of PDA | Targeted and imaging-guided PTT/PDT: Enhanced photothermal conversion efficiency resulting from the association of PDA and ICG, Enhanced cell killing effect of PTT/PDT combination in comparison to single treatment modality | PTT/PDT: 808 nm, 1.2 W cm−2, 5 min (for in vitro tests) | In vitro: MDA-MB-231 cells (breast cancer) | [253] |
| Gold nanorods (GNRs)@PDA shell-MB-PEG NPs | — | Methylene blue (MB) | Enhanced cellular uptake of MB | PTT/PDT: Enhanced photothermal conversion efficiency resulting from the association of PDA and GNRs, Synergistic therapeutic effects of PTT/PDT combination in vitro and in vivo in comparison to single treatment modality | PTT: 808 nm, 2 W cm−2, 5 min (for in vitro and in vivo tests);PDT: 671 nm, 30 mW cm−2, 3 min (for in vitro and in vivo tests) | In vitro: HeLa cellsIn vivo: HeLa-bearing mice | [138] |
| IR820/Fe3+-PDA-PEG NPs | — | IR820 | Dual imaging: photoacoustic (PA)- and magnetic resonance (MR)-guided imaging | Imaging-guided PTT/PDT: Enhanced cell killing effect of PTT/PDT combination in comparison to single treatment modality | PTT/PDT: 808 nm, 1 W cm−2, 10 min (for in vitro tests) | In vitro: HeLa cells | [254] |
| PDA@PS-HA shell | Hyaluronic acid (HA) | Pheophorbide a (Pheo a) | Tumor targetingHyaluronisase-responsive Pheo a release | Targeted PTT/PDT: Enhanced therapeutic effects of PTT/PDT combination in vitro and in vivo in comparison to single treatment modality | PTT/PDT: 670 nm laser, 3 J cm−2 (for in vitro tests) and 100 J cm−2 (for in vivo tests) | In vitro: MDA-MB-231 cells (breast cancer)In vivo: MDA-MB-231-bearing mice | [255] |
| TPGS micelles @ PDA nanoclusters | — | IR780 | Overcoming tumor drug resistanceTemporally controlled sequential system activation by consecutive light irradiations NIR light-responsive DOX release | PTT/PDT/CT: Complementary interactions and synergistic therapeutic effects of PTT/PDT/CT combination in vitro and in vivo in comparison to single treatment modality, for multi-drug resistant tumors | PTT/PDT: 808 nm, 0.5 W cm−2, 10 min (for in vitro tests) and 5 min (for in vivo tests) | In vitro: MCF-7/ADR cells (breast cancer)in vivo: MCF-7/ADR -bearing mice | [256] |
| ZnPc-FA-PDA NPs | FA | ZnPcpH-responsive release of ZnPc | Tumor targeting | Targeted PDT | PDT: 680 nm, 5 J cm−2 for 2 min (for in vitro tests) or 75 J cm−2 for 10 min (for in vivo tests) | In vitro: HeLa cells, MCF-7 cellsin vivo: HeLa or MCF-7-bearing mice | [172] |
| ZnPc-NOC-PDA NPs | — | ZnPc,Nocodazole (NOC) | pH-responsive release of ZnPc and NOCEnhanced nuclear uptake of ZnP by NOC, Fluorescent molecular tomography imaging capability | Cell proliferation inhibition/PDT: Synergistic therapeutic effects of cell proliferation inhibition/PDT combination in vitro and in vivo in comparison to single treatment modality | PDT: 680 nm, 7.5 J cm−2 for 3 min (for in vitro tests) or 50 J cm−2 for 5 min (for in vivo tests) | in vitro: MCF-7 cellsIn vivo: MCF-7-bearing mice | [257] |
| CaCO3-PDA-PEG NCs | — | Ce6 | MR-guided imagingpH-responsive of Ce6, Reduced skin phototoxicity | MRI-guided PDT | PDT: 660 nm light emitting diode (LED), 5 mW cm−2, 1 h (for in vivo tests). | In vitro: 4T1 cellsIn vivo: 4T1 cells-bearing mice | [76] |
| PDA NPs | — | — | ΔT = 34 °C for 200 µg ml−1 under 808 nm, 2 W cm−2, 500 sPhotothermal conversion efficiency of ∼40% | PTT | PTT: 808 nm, 2 W cm−2, for 5 min (for in vivo tests) | In vitro: HeLa cells, 4T1 cellsIn vivo: 4T1 cells-bearing mice | [46] |
| PDA-Fe3+-PEG NPs | — | — | ΔT ∼30 °C for 250 µg ml−1 under 808 nm, 1.3 W cm−2, for 10 minpH-activatable MRI contrast | MRI-guided PTT | PTT: 808 nm, 1.3 W cm−2, for 6 min (for in vivo tests) | In vitro: SW620 cells (colon cancer)In vivo: SW620-bearing mice | [54] |
| PEG-PDA/mesoporous Ca phosphate hollow JNPs | — | DOXICG | pH- and NIR irradiation-responsive releasePA imaging | PA-guided PTT/CT: Enhanced photothermal conversion efficiency resulting from the association of PDA and ICG, Enhanced therapeutic effects of PTT/CT combination in vitro and in vivo in comparison to single treatment modality | PTT: 808 nm NIR laser, 1 W cm−2 (for in vivo tests) | In vitro: HepG2 cellsIn vivo: HepG2-bearing mice | [65] |
| PNAm/PDA-coated AuNPs supramolecular polymer nanocomposite hydrogel | — | DOX | pH- and heat-responsive drug release. | PTT/CT: Enhanced photothermal conversion efficiency resulting from the association of PDA and Au NPs, Synergistic therapeutic effects of PTT/CT combination in vitro and in vivo in comparison to single treatment modality | PTT: 808 nm NIR laser, 2 W cm−2 (for in vitro and in vivo tests) | In vitro: L929 cells (murine fibroblast cells)In vivo: 4T1-bearing mice (breast cancer model) | [258] |
| Cu/PDA NPs | — | Cu2+ | ΔT = 42.2 °C for 75 µg ml−1, under 808 nm, 3.5 W cm−2, for 20 minpH-responsive Cu2+ release Amplified NIR absorption of PDA in the presence of Cu2+ MRI guidance | MRI-guided PTT/CT: Enhanced photothermal conversion efficiency resulting from the association of PDA and copper, Synergistic therapeutic effects of PTT/CT combination in vitro and in vivo in comparison to single treatment modality | PTT: 808 nm, 1 W cm−2, for 10 min (for in vitro tests), 0.33 W cm−2, 20 min (for in vivo tests) | In vitro: KB cells (epidermal cancer)In vivo: KB-bearing mice | [259] |
| Selenide molybdenum (MoSe2) nanosheets (NSs)@PDA | — | DOX | pH- and heat-responsive drug release | PTT/CT: Enhanced photothermal conversion efficiency resulting from the association of PDA and MoSe2 NSs, Synergistic therapeutic effects of PTT/CT combination in vitro and in vivo in comparison to single treatment modality | PTT: 808 nm, 2 W cm−2, for 5 min (for in vitro and in vivo tests) | In vitro: HeLa cellsIn vivo: U14-bearing mice (cervical cancer model) | [260] |
| Black phosphorus nanosheets (BP NSs)@PDA-PEG-Aptamer | Aptamer | DOXP-gp siRNA | Tumor targeting,pH- and heat-responsive drug release | Targeted PTT/CT/gene therapy: Enhanced photothermal conversion efficiency resulting from the association of PDA and BP NSs, Synergistic therapeutic effects of PTT/CT/gene therapy combination in vitro and in vivo in comparison to single treatment modality, for multi-drug resistant tumors | PTT: 808 nm, 1.5 W cm−2, for 5 min (for in vivo tests) | In vitro: MCF-7 cellsIn vivo: MCF-7-bearing mice | [261] |
FRET: fluorescence resonance energy transfer, GO: graphene oxide, CT: chemotherapy, JNPs: Janus nanoparticles, NCs: nanocapsules, PNAm; poly(N-acryloyl glycinamide-co-acrylamide), TPGS: D-α-tocopheryl poly(ethylene glycol) 1000 succinate.
5.3.1. PDA nanoparticles for PTT
Liu et al demonstrated the high photothermal efficiency of PEGylated PDA nanoparticles in vitro and in vivo, on a colon cancer model [54]. Significant tumor growth suppression was obtained following NPs intravenous injection into SW620-bearing mice and illumination using an 808 nm laser at 1.3 W cm−2 for 6 min.
Various strategies have been employed to further enhance the photothermal effects obtained with PDA for an improved therapeutic efficacy.
Light absorbing molecules like ICG or IR820, can act as 'thermal-enhancement agents' [262]. They would result in an increased NIR absorption and their association to PDA nanomaterials has been described in several studies and resulted in enhanced photothermal performance and therapeutic outcomes. For instance, the association of the FDA-approved photosensitizing molecule ICG with PDA has been investigated for PAI-guided photothermal cancer treatment [226, 263]. The loading of ICG into PDA allowed to significantly increase the system's NIR light absorption, up to 13 times higher at 780 nm. In return, PDA quenched the fluorescence of ICG and prevented its photobleaching, and lead to an improved stability under its laser irradiation. The hybrid nanoplatform exhibited an amplified photothermal effect with a temperature increment exceeding 14 °C in presence of ICG in comparison to the pristine system (after illumination at 808 nm, 0.6 W cm−2 for 5 min, 20 µg ml−1), as well as an improved photoacoustic contrast (figure 9). The nanosystem represented hence a promising phototheranostic nanoagent achieving a complete tumor suppression with a low laser density, as evidenced by in vivo experiments.
Figure 9. ICG-loaded polydopamine-reduced graphene oxide (rGO) nanocomposites with amplified photothermal and photoacoustic effects for cancer theranostics. (A) Schematic illustration of the preparation process of ICG-PDA-rGO. (B) Photothermal heating curves of PBS, GO, PDA-rGO and ICG-PDA-rGO solutions, [GO] = [PDA-rGO] = [ICG-PDA-rGO] = 20 mg l−1. (C) Photothermal heating curves of ICG-PDA-rGO solutions of different concentrations. (D) Temperature evaluation of ICG-PDA-rGO and equal free ICG solutions over four laser ON/OFF cycles (laser ON time: 5 min, laser OFF time: 5 min) (E) UV–vis absorption spectra of free ICG and ICG-PDA-rGO solutions before and after four cycles of laser irradiation at 808 nm and 0.6 W cm−2. Reproduced with permission from [226]. © Ivyspring International Publisher.
Download figure:
Standard image High-resolution imageThe coupling of metal ions, like Cu2+ and Mn2+, to PDA nanostructures allows not only to perform bio-imaging but also to achieve amplified photothermal effects [34, 259]. Cu2+-loaded PDA nanoparticles were proposed by Ge et al as an efficient MRI-guided therapeutic nanoagent [259]. Indeed, in presence of Cu2+, a four-fold increase in PDA nanoparticles molar extinction coefficient was obtained, accompanied with a significantly enhanced photothermal conversion efficiency reaching 54.8% for CuPDA against 37.6% for PDA nanoparticles of a similar size (51 nm). Laser irradiation of a 75 µg ml−1 nanoparticles suspension at 808 nm and 3.5 W cm−2 induced a temperature increase of 21.5 °C for PDA versus 42.2 °C for CuPDA nanoparticles. Interestingly, the nanocomposite displayed a pH-responsive release of copper ions allowing their efficient delivery in the tumor tissue. The released ions exhibited a synergistic chemotherapeutic cancer cells killing effect.
Enhanced photothermal effects could also be obtained through the combination of PDA nanostructures to other photothermal converting agents. In this perspective, PDA-Au nanohybrids represent promising materials that benefit from the plasmonic photothermal activity of gold nanosystems along with the interesting photothermal conversion properties of PDA. For instance, Wu et al fabricated an injectable thermoresponsive poly(N-acryloyl glycinamide-co-acrylamide) hydrogel containing PDA-coated gold nanoparticles and the anti-cancer drug doxorubicin, and serving as a breast filler for efficient prevention of breast cancer recurrence [258]. This nanocomposite exhibited an intriguing photothermal activity arising from the combination of both gold nanoparticles and PDA coating. Interestingly, a red shifting was observed in the gold nanoparticles absorption spectra upon modification with PDA, suggesting a higher photothermal efficiency. A pronounced temperature increase was obtained, with a ΔT reaching 43 °C after a 5 min irradiation with a 808 nm laser at 2 W cm−2.
In another example, the biocompatible graphene oxide (GO) was utilized in association with PDA, owing to its intrinsic high NIR light absorption and photothermal conversion ability. It was suggested that PDA would restore a part of π conjugation in GO through the reduction of this latter, generating stable reduced GO (rGO), characterized by its higher NIR light absorption and enhanced photothermal activity. This association is thereby very suitable for PTT applications, explaining the interesting temperature increase revealed in several studies for the PDA/GO nanohybrids [174, 226, 251].
5.3.2. PDA as nanocarriers of photosensitizers for PDT applications
PDT is a potent light-based therapeutic modality clinically approved for cancer treatment [264] and is based on the use of a photosensitizer (PS), a light-absorbing compound, which photo-activation at an appropriate wavelength, leads eventually to the generation of ROS that are cytotoxic. Indeed, following the PS administration, the near infrared (NIR) irradiation of the tumor site induces the photo-excitation of the PS to an excited singlet state. The excited PS can then re-gain its fundamental state either directly through fluorescence emitting or via conversion to a triplet state, a lower energy excited state, through an intersystem crossing. At this state, the PS can interact either with nearby substrates through electron transfer to eventually produce free radicals (type I PDT reactions) or with molecular oxygen through energy transfer to generate singlet oxygen that is highly cytotoxic (type II PDT reactions) [224]. Although highly promising, PSs usually suffer from poor water solubility limiting their bioavailability and therapeutic efficacy, which renders their encapsulation and administration using nanocarriers interesting. These latter allow to considerably improve the biodistribution of photosensitizers and enhance their membrane permeability, resulting in enhanced therapeutic efficacy and minimal side effects. In this context, PDA-based nanostructures have emerged as promising nanocarriers for photosensitizing agents and have thus been largely applied in anti-cancer PDT. For example, taking advantage of the negative charge of PDA, Yan et al synthesized a highly positively charged zinc-phthalocyanine, the ZnPc(TAP)4 12+, which allowed the physical adsorption of this water-soluble phthalocyanine derivative, on the surface of PDA nanoparticles via electrostatic interactions. The nanosystem was further modified with a tumor targeting moiety, folic acid (FA), which offered a high tumor accumulation and resulted in specific and efficient cancer cells eradication [172]. A resembling system was previously proposed by the same group of researchers in which both ZnPc(TAP)4 12+and nocodazole (NOC), a cell cycle inhibitor, were immobilized on PDA nanoparticles surface using electrostatic interactions for an enhanced synergistic anti-cancer therapy [257].
Another promising nanomedicine was proposed by Liu et al who developed an innovative oxygen-self supplied platform composed of a photosensitizer, methylene blue, carried by PDA-hemoglobin complexes encapsulated inside a bio-vesicle engineered from recombined red blood cells (RBCs) membranes and serving as oxygen carrier and supplier [265]. In fact, with oxygen being crucial for the photodynamic effects, tumors extreme hypoxia represents a major mechanism of resistance to the PDT. To bypass this bio-barrier, the corpuscular hemoglobin in this nanosystem was about ten-fold higher than that of natural RBCs, thanks to the abundant catechol functions present in PDA. Beside its role as a drug carrier, the anti-oxidation property of PDA, which is ascribed to the strong anti-oxidation ability of polyphenols, allowed it to mimic the anti-oxidative enzymes in RBCs, preventing hence the oxidative degradation of oxygen-transporting hemoglobin. This platform demonstrated its high potential for inducing a strong PDT effect against normoxic or extremely hypoxic tumors leading to a complete tumor ablation. It can interestingly serve as a universal platform for other oxygen-involved therapies such as radiotherapy.
5.3.3. PDA/PS as synergistic photothermal/photodynamic nanoplatforms
PTT/PDT bimodal therapy has emerged as a promising non-invasive approach that has shown encouraging results in the cancer treatment field. In fact, this combination is based on the generally accepted synergistic effects obtained by associating both modalities. On one hand, the hyperthermia generated by the photothermal converting agent can enhance blood flow into the illuminated tumor site, which leads to an improved tumor oxygenation and consequently an enhanced concurred oxygen-mediated PDT effect [262]. Moreover, the local hyperthermia would induce membrane permeabilization, thus increasing cellular uptake of the photosensitizing agent or generated singlet oxygen [251]. On the other hand, the oxygen depletion taking place in the tumor microenvironment during PDT renders the cells more sensitive to heat, enhancing thereby the efficacy of PTT [246]. In addition, both phototherapeutic modalities share several advantages and drawbacks as depicted in figure 10 and may be complementary to each other.
Figure 10. Key factors involved in photodynamic therapy (PDT) and photothermal therapy (PTT) as described in reference [262].
Download figure:
Standard image High-resolution imageIn this perspective, many works have pointed out the great potential of combining PDA nanomaterials to PS in the aim to design of multifunctional materials with improved therapeutic outcomes through the combination photothermal and photodynamic effects. Curiously, based on the broad absorption band of PDA, single-wavelength activation protocols were applied in several studies in this approach [185], which facilitates the illumination procedure by reducing the needed equipment, and would lead to enhanced therapeutic efficacy due to the synchronized PTT/PDT activities [30]. Nevertheless, PDA activation for PTT usually uses longer wavelength irradiations (808 nm) in comparison to conventional wavelengths used in PDT, which permits to reach higher tissue-penetration depth and eradicate deeper tumoral tissues.
Several PSs have been coupled (scheme 2) to PDA nanoconstructs for the development of PDA-based bifunctional nanoplatforms. This can be done by using various strategies of PS couplings depending on the therapeutical application. Indeed, the PS can be (a) loaded inside the PDA matrix, (b) physically adsorbed on the surface of nanoparticles or (c) covalently bound to their surface (figure 11).
Scheme 2. Structures of photosensitizers used for the design of PDA-based nanoparticles for synergistic photothermal/photodynamic therapy.
Download figure:
Standard image High-resolution imageFigure 11. (A) PDA/PS nanoplatforms for synergistic photothermal/photodynamic therapy. (B) Strategies of PS coupling to PDA nanostructures.
Download figure:
Standard image High-resolution image5.3.3.1. PS loaded inside PDA matrix
Taking advantage of the abundance of aromatic groups in PDA, Poinard et al developed a photothermal/photodynamic therapeutic nanoagent based on PDA nanoparticles encapsulating the hydrophobic PS chlorin e6 (Ce6) within the polymeric core, via π–π stacking interactions [185]. This strategy allowed to load unprecedently high amounts of Ce6 in the PDA-based nanocarrier and offered an enhanced cellular uptake (over eight folds higher) with a sustained drug release up to 5 d. Under a single laser irradiation at 665 nm and at a relatively low power density of 250 mW cm−2, this nanosystem showed improved additive cell killing effects in comparison to PTT or PDT alone.
Due to the fluorescence quenching and ROS scavenging abilities of PDA [26, 266], the encapsulation of the photosensitizing drug in the core of the nanoparticles would prevent its degradation and limit its undesirable activation under day light. However, ROS generation and subsequently the anti-cancer photodynamic effect is only correlated to the amounts of released PS [185]; the unreleased PS remains inactive. Moreover, although this encapsulation strategy allows to preserve the surface properties of the nanosystem and despite the high PS loading amounts and the efficient drug delivery to the tumor, drug embedment in PDA matrix may induce changes in the stacking and aggregation of PDA oligomeric building units, which would consequently impact the photophysical properties of PDA nanosystems and their nanomechanics, compromising hence their biological performance and therapeutic efficacy.
5.3.3.2. PS adsorbed onto the surface of PDA nanomaterials
Diverse physical interactions could be deployed for the loading of PS on PDA, particularly via π–π interactions, hydrophobic interactions, hydrogen bonding or electrostatic interactions. Multiple PS-bearing PDA nanosystems have been described for this category.
Wang et al proposed a multifunctional drug delivery platform, based on PDA-coated gold nanorods (GNRs), for multi-modal cancer theranostics [138]. The nanohybrid was further functionalized with PEG which increased its colloidal stability. A photosensitizer, methylene blue (MB), was then adsorbed on the surface of PDA via electrostatic and π–π interactions. The association of GNRs to PDA resulted in an improved PTT performance which allowed an efficient tumor ablation, as demonstrated in vitro and in vivo. The combination of hyperthermia and ROS generation related to MB, promoted the anti-cancer therapeutic efficacy.
Similarly, IR820, a NIR light absorber with a great potential in photodynamic and photothermal therapies but also in PAI, was used in association with PDA after being adsorbed on the surface of PEGylated PDA nanoparticles via π–π and electrostatic interactions [254]. The combination of both photothermal agents offered a considerable enhancement in the light-heat conversion efficiency of the nanoagent with a great photostability, which together with the ROS generation ability of the IR820, allowed an efficient cancer treatment following a single laser irradiation at 808 nm with a power density of 1 W cm−2. Moreover, given the interesting photoacoustic properties of PDA nanoparticles and IR820, this system showed a great potential as a multifunctional nanoplatform for cancer theranostic applications.
A tumor targeting PTT/PDT platform was developed by Han et al for improved therapeutic specificity and consisted in a novel photomedicine composed of a PDA core and a pheophorbide a-hyaluronic acid shell as an efficient anti-cancer agent [255]. While hyaluronic acid acts as the tumor-targeting moiety, PDA acts as a quencher hindering the fluorescence and singlet oxygen generation of the conjugated PS. However, in the presence of cancer-specific enzymes, particularly hyaluronidase, the PS is released and its photo-mediated activity is restored, leading to a localized photodynamic effect. Following a single laser irradiation at 607 nm, the nanosystem exhibited a synergistic PTT/PDT anti-tumoral activity and showed promising results in vitro and in vivo.
In a more recent study, Chen et al proposed a novel PDA-based PTT/PDT dual platform in which aggregation-induced emission (AIE) gens were used as efficient photosensitizing agents [252]. Herein, a mitochondria-targeting AIE PS conjugate was attached to the surface of PDA nanoparticles via π–π interactions, H-bonding and electrostatic attraction, and showed an unprecedently high loading capacity with a low leakage rate. A synergistic PTT/PDT-mediated tumor inhibition was indeed obtained in vitro and in vivo with low IC50 of 71 µg ml−1 versus 170 µg ml−1 or 105 µg ml−1 for PDT (white light, 100 mW cm−2) or PTT (808 nm laser, 1 W cm−2) alone, respectively. The nanohybrid system displayed a positive surface charge which resulted in pronounced cytotoxic effects.
The physical adsorption of the photosensitizing molecules on the surface of PDA nanoparticles or shells uses relatively simple chemical procedures for molecules immobilization, in comparison to covalent conjugation. Although such interactions are energetically weak, studies have demonstrated their relative stability under physiological conditions. They are on the other hand easily weakened under the acidic, reductive or oxidative conditions characterizing the tumor microenvironment or under NIR irradiation, which offers an efficient PS delivery at the targeted site. Premature leakage of the PS from PDA surface could however be observed limiting consequently the therapeutic efficiency and increasing the side effects. Improved stability may hence be achieved through covalent coupling of the PS to the nanostructure.
5.3.3.3. PS covalently bound to the surface of PDA nanomaterials
Zhang et al reported the preparation of Ce6-carrying PDA nanospheres via covalent binding between the carboxylic acid of the PS and the amine groups exposed on PDA surface, using carbodiimide chemistry [179]. The combined photothermal and ROS-mediated effects of PDA and Ce6 respectively, resulted in a significantly enhanced anti-cancer therapeutic efficacy as demonstrated through in vitro and in vivo studies, following a dual-wavelength laser irradiation (808 nm for PDA activation and 670 nm for Ce6) [179].
This carbodiimide-mediated conjugation strategy has been later employed by Wang et al for the immobilization of the Ce6 on the surface of PDA nanoparticles. However, in order to improve the selectivity and therapeutic efficacy of this nanomedicine, the authors decorated herein the Ce6-bearing PDA nanospheres with a tumor targeting moiety, hyaluronic acid (HA), which offered a remarkably higher tumor penetration and resulted in a superior synergistic anti-tumor effect in comparison to the non-targeting platform [246].
In another approach, Yang et al [248] selected black phosphorus (BP) nanosheets (NSs), biocompatible and biodegradable inorganic materials, as the nanocarrier for Ce6 delivery, taking advantage of its high surface area that offers considerably high loading amounts of the PS. PDA was used here to coat the NSs and served as a bridge to enable the covalent anchoring of Ce6 and triphenyl phosphonium (TPP), a mitochondria-targeting moiety, using carbodiimide chemistry for both reactions. Under a single 660 nm irradiation, the combination of PDA and BP in the nanosystem resulted in an increased photothermal conversion efficiency, with 33.2% for PDA@BP NSs versus 26.6% for BP NSs alone, while Ce6 induced efficient ROS generation and photodynamic effect. The as-prepared platform exhibited a selective localization in mitochondria which further promoted the phototherapeutic efficiency, as demonstrated in in vitro and in vivo cancer models.
Other covalent interactions were engaged for the design of similar bimodal photoactivatable nanoagents. For instance, Hu et al have developed C60-PDA-graphene nanohybrids as a promising anti-cancer candidate for synergistic PTT/PDT [251]. In this study, fullerene C60, a promising photodynamic agent, was conjugated to the targeting moiety folic acid (FA), and the resulting conjugate was anchored on PDA-rGO nanohybrid using nucleophilic Michael addition or Schiff base reaction of the amine-terminated FA on the o-quinone function of PDA. The covalent attachment of C60-FA conjugate on the nanohybrid structure limited the aggregation of the former observed in previous studies, preserving its photodynamic activity. As mentioned above, the association of PDA with rGO results in enhanced NIR light absorption and subsequently in improved heat generation upon light activation. Interestingly, the C60-FA conjugate contributed to the global photothermal activity of the nanosystem, with an induced temperature elevation of 7.5 °C versus 17.5 °C for PDA-rGO hybrid after irradiation with a Xe lamp at 2 W cm−2 for 15 min at a concentration of 50 µg ml−1. After a single irradiation using the Xe lamp, the nanoplatform showed efficient photo-induced apoptotic effects related to a synergistic PTT/PDT anti-tumoral activity.
In order to achieve a superior treatment efficacy and ensure its biosafety, it is highly desirable to design smart nanocarriers that specifically deliver the PS to the targeted tumor tissue and in a controlled manner. In this context, a smart PTT/PDT controllable platform was proposed by Zhan et al based on the complementary base pairing rules [267]. A thymine-modified zinc phtalocyanine (T-ZnPc) was bound to adenine-modified PDA nanoparticles via the base pairing rules. The hyperthermia induced by PDA photo-activation will break the base pairing resulting in a NIR-triggered release of the ZnPc. Synergistic photodynamic/photothermal effects allowed efficient cancer ablation.
The covalent conjugation of the PS offers a higher stability and biosafety to the nanomedicine and allows an efficient delivery of the molecule to the targeted site. The design of smart stimuli-responsive delivery systems allows to further control the release behavior of the PS for a higher efficiency. Nonetheless, more efforts are still needed for the development of such systems, through the exploration of the potential of diverse smart cleavable linkers, which would offer a spatio-temporally controlled release under external stimuli.
Although the excellent activity of these systems has been largely demonstrated through in vitro and in vivo models, the direct immobilization of the PS on PDA results in a partial decrease in the PS-mediated ROS generation [248, 251], due to the redox activity of PDA and its ROS scavenging capacity. This issue should be considered by coupling the PS to PDA nanoplatforms using hydrophilic polymers, such as polyethylene glycol.
5.4. Multi-modal cancer therapy
In order to reach better therapeutic outcomes, multi-modal cancer therapy is widely adopted for cancer treatment. Diverse all-in-one innovative PDA-based platforms have been described in the literature as multifunctional/multi-modal therapeutic or theranostic nanoagents [142, 256, 268]. Numerous reviews published over the recent years have cited in great details the diverse therapeutic approaches and associations regarding PDA-mediated anti-cancer treatment [29–32, 148, 269].
6. Other catecholamines-derived melanin-like materials
The outstanding properties of PDA-based materials and their remarkable potential in diverse biomedical applications, particularly in the cancer therapy field, have boosted the research of new melanin-like materials for the design of novel therapeutic agents. Catecholamines like 3,4-dihydroxy-L-phenylalanine (L-DOPA) or norepinephrine (scheme 3), and other related molecules like serotonin (scheme 3), have been considered for the development of bioinspired advanced functional materials. Other naturally occurring catechols or dopamine chemical derivatives [23, 24, 149] were also described in the literature for the preparation of PDA-resembling materials. Natural melanin granules have also gained interest as photothermal and photoacoustic agents showing high photothermal conversion efficiency (about 40%) and excellent anti-cancer activity revealed through in vitro and in vivo assays [270–272].
Scheme 3. Structures of the main monomers explored for the synthesis of polydopamine-resembling materials.
Download figure:
Standard image High-resolution imageThe small structural differences between precursor monomers may result in a divergence in the functional properties and biological performance of the corresponding polymerized structures, which would offer a rich repertoire of melanin-mimicking materials of tailored characteristics.
Here, we will describe the ability of three main precursors, namely L-DOPA, norepinephrine and serotonin, to give rise to PDA-like 'polymers' following their oxidation.
6.1. Poly(L-DOPA) (PDOPA)
3,4-Dihydroxy-L-phenylalanine or L-DOPA is decarboxylated in vivo to generate dopamine. As the natural precursor of eumelanin in vivo [273], L-DOPA is the first obvious material that can be considered for the preparation of synthetic melanin structures.
Inspired by the large advancements achieved for PDA development, PDOPA coating materials and colloidal nanoparticles were successfully prepared using the solution oxidation method [274], the enzyme-catalyzed polymerization process [275, 276] or also the electro-polymerization method [277]. Nevertheless, the formation of PDOPA can be less successful than PDA, probably because of the electrostatic repulsion occurring between neighboring carboxylic acid groups, hindering the stacking and the aggregation of oligomeric units. This limitation could be however overcome under high ionic strength conditions [278]. Differences in the oxidation and polymerization rates were found for L-DOPA in comparison to dopamine, which lead to different deposition rates and particles formation kinetics [279].
Adding to the reactive sites described for PDA in section 4.2.3, the chemical reactivity of PDOPA takes advantage of the carboxylic acid group available for diverse secondary reactions including with amine or hydroxyl-bearing molecules [279, 280]. Also, bridging the carboxylic functions of L-DOPA monomers via a disulfide bond allowed to prepare novel PDOPA coatings offering an on-demand degradation in presence of reducing agents like glutathione and was applied for the delivery of doxorubicin in tumor microenvironment [279, 281].
PDOPA coatings were in fact successfully deposited on diverse surfaces such as metals, polymers, oxides and emulsion droplets [113, 278], and were used for diverse biomedical purposes, including cancer therapy. For instance, novel magnetic-guided PDOPA-coated Fe3O4 nanoparticles were recently developed for targeted delivery of the anti-cancer drug taxol and showed a promising tumor growth inhibition effect in vivo, with a more uniform drug release profile in comparison to PDA-coated analogue structures [282].
In comparison to PDA nanoparticles, PDOPA counterparts displayed a stronger NIR light absorption and exhibited an enhanced photothermal conversion efficiency under laser illumination at 808 nm and a power density of 2 W cm−2 [274]. These properties can be advanced to develop photothermal therapeutic nanoagents with a great potential in cancer treatment applications.
6.2. Poly(norepinephrine) (PNE)
Norepinephrine is another endogenous catecholamine that, similarly to the parent dopamine and L-DOPA, can spontaneously polymerize under ambient alkaline conditions. The synthesis of PNE was first reported in 2009 after PDA discovery [283]. PNE was at first mostly reported as a versatile coating material applied to coat substrates of different types and shapes including nanoparticles [284], nanotubes [285] and nanofibers [286] and could deposit on the surface of several organic and inorganic substrates including polymers, metals, silicates and GOs [287, 288].
Following the protocols described for PDA formulation, spherical monodisperse PNE nanoparticles were successfully prepared, using a NaOH solution [289] or an alkaline Tris buffer/ethanol mixture [290]. Their size and morphology were tunable by varying the monomer or solvent amounts. For instance, increasing the monomer amount led to the increase of nanoparticles size. The increase of ethanol concentration allowed the obtention of nanoparticles of smaller size, while the use of ethanol at very low concentrations (0.1 mM) induced a loss in their spherical shape and monodispersity, which was attributed to the Hansen solubility parameter theory, as for PDA formation.
Similar to PDA, PNE materials expose multiple reactive sites serving as anchors for the immobilization of diverse biomolecules (enzymes [285, 291, 292], antibodies [293], etc) and for nanoparticles functionalization using PEG chains [289].
In comparison to its PDA analogue, conventional PNE present an ultra-smooth and a highly homogeneous surface, due to the presence of 3,4-dihydroxybenzaldehyde, an intermediate generated during the polymerization of norepinephrine [294]. This property is advantageous for biomedical applications and allows to overcome limited surface regularity encountered in PDA [287]. Indeed, PNE nanoparticles showed increased hydrophilicity and enhanced anti-fouling properties in comparison to their PDA counterparts suggesting a superior potential in biological applications [290] and PNE coatings served as an interesting bio-interface used to endow coated biomaterials with high hydrophilic and anti-fouling properties [290]. Moreover, following the immobilization of an enzyme on the PDA or PNE surfaces, higher enzyme loading rates were obtained for PDA due to this latter's rougher surface, however a decreased enzyme activity was observed and attributed to a reduced accessibility of its active sites [285]. The higher hydrophilicity of PNE counterparts allowed on the other hand to promote the activity of the immobilized enzyme.
Owing to its bioinspired nature and this highly hydrophilic character, PNE nanoparticles exhibited excellent biocompatibility as demonstrated in in vitro and in vivo studies and showed no immune response or long-term toxic effects during in vivo studies [289, 290, 295, 296].
Considering all these aspects, PNE nanomaterials have gained increasing attention for biomedical applications, including tissue engineering [297] and gene delivery [298] applications, and have recently emerged as smart thermal and/or pH-responsive drug delivery platforms and excellent photothermal nano-agents, applied for combined chemo-PTT cancer therapy.
Indeed, due to its composition rich in aromatic rings, PNE nanoparticles are able to load compounds with aromatic rings, like doxorubicin, and hold a great potential as anti-cancer drugs nanocarriers [289, 299]. Interestingly, PNE nanoparticles showed excellent drug loading ability with a maximum loading capacity of DOX that exceeded greatly that of PDA counterparts. Besides, PNE nanoparticles exhibited a higher cellular uptake efficiency, which resulted in a significantly enhanced intracellular DOX accumulation in comparison to PDA, confirming hence their superior potential in drug delivery applications. Similar to PDA, PNE nanomaterials showed a pH-sensitivity leading to a reduced leakage during blood circulation and to an enhanced drug release in acidic tumor microenvironment and cytolysosome compartments, due to the protonation of the amine functions in PNE causing the disruption of the π–π interactions.
Moreover, very recently, PNE nanomaterials were revealed to be a promising photothermal converting nanoagent of a great potential in cancer therapy. Indeed, PNE nanoparticles displayed a high NIR light absorption and an excellent photothermal conversion efficiency of 67% for nanoparticles of ∼100 nm, following their irradiation at 808 nm, which is a 1.63-fold higher than that of their PDA analogues (41%), and higher than most available PTT nanoagents [289]. A great photostability was also observed in the case of PNE nanoparticles upon repeated laser illumination. The therapeutic efficacy of PNE nanoparticles was demonstrated through in vitro cells and in vivo experiments. Interestingly, under nanoparticles illumination, increased release of encapsulated DOX was obtained, probably due to nanoparticles thermal expansion. This NIR-responsive drug release offered a further control of the site-specific delivery of anti-cancer drugs and results in a synergistic chemo-photothermal effect for an efficient tumor ablation.
Similarly, PNE coatings have shown promising photothermal activity and drug loading ability and were explored for the development of novel artemisinin-loaded PNE-coated FeOOH nanoparticles for efficient cancer photothermal-chemical combination therapy [299].
6.3. Poly(serotonin) (PST)
PST is a less explored class of melanin-like material obtained from the oxidative self-polymerization of serotonin, an indoleamine neurotransmitter derived from the hydroxylation and decarboxylation of the amino acid tryptophan.
The polymerization process, conducted under alkaline conditions, generated monodisperse nanoparticles, whose formation occurred with significantly slower kinetics compared to PDA, as monitored using absorption spectroscopy [300].
The drug loading ability, stimuli-responsiveness and photothermal activity of PST nanoparticles have been recently investigated. The anticancer drug DOX was tested and could be efficiently loaded on the surface of PST nanoparticles via π–π and hydrophobic interactions and through hydrogen bonds formation. However, the drug loading efficiency was found to be lower than that of PDA nanoparticles. A controlled pH-induced release of the chemotherapeutic drug was depicted, confirming the interest of PST-based nanosystems in drug delivery applications for tumor therapy.
Moreover, the illumination of PST nanoparticles suspensions at 808 nm and 1.5 W cm−2 induced a solution temperature increase of >20 °C for nanoparticles concentrations >100 µg ml−1. The temperature increase generated by PST nanoparticles was however lower than that obtained for their PDA counterparts.
Nevertheless, although the photothermal activity and drug loading capability of PST nanoparticles were limited compared to PDA materials, the former represent promising anticancer nanoagent candidates. Particularly, PST nanoparticles showed a greater biocompatibility, and a reduced protein corona formation in comparison to PDA, which is highly interesting for in vivo applications. Indeed, at the nano-bio-interface polyserotonin nanoparticles showed reduced interactions with proteins corona as suggested by quantum mechanics computations, which was attributed to the four-foldlower adhesion obtained for PST nanoparticles compared to PDA ones during peak-force AFM experiments.
Based on these findings, PST-based nanoparticles can be used as multifunctional cancer therapeutic nanoconstructs either as photothermal agents or as drug nanocarriers for efficient anticancer therapy.
7. Summary and outlooks
In summary, PDA represents a successful example of nature-inspired materials. Considering its diverse inherent properties, including the excellent biocompatibility, the high chemical reactivity and the remarkable photothermal conversion ability, PDA is acknowledged as a robust element that can be considered for the development of innovative 'all in one' platforms, in order to provide an efficient cancer treatment and diagnosis.
Nevertheless, despite the rapidly increasing implementation of PDA and catecholamines derivatives in nanomedicines research field, several remaining challenges still have to be overcome to achieve advancements towards future clinical translation.
- Regarding the complicated formation mechanisms, thorough investigation based on advanced techniques should be undertaken for a better understanding of the molecular organization and assembly modes. This would offer a full control of the morphological and functional features of PDA materials, allowing consequently the expansion of their applications scope.
- The purification methods described so far to separate PDA nanoparticles from the oligomeric and unpolymerized counterparts are mostly based on dialysis or centrifugal filtration. However, these latter seem to be inefficient to separate all of the oligomeric structures from PDA nanoparticles and especially when it comes to nanoparticles with a hydrodynamic diameter less than 100 nm. The presence of such oligomeric structures in PDA nanoparticles suspension would affect their physicochemical properties and their in vivo outcomes.
- As the preparation method leads to different chemical structures of PDA assemblies, this may affect their physicochemical properties and thus their interaction with biological environment. Hence, the preparation procedures should be harmonized between the different studies in order to get a relevant comparative set of data regarding the in vitro or in vivo studies.
- Full comprehensive studies of the photothermal conversion efficiencies of PDA nanostructures as a function of particles size and preparation procedures are also still lacking.
- Moreover, due to the high chemical reactivity and sensitivity of PDA structures, precautions need to be taken during formulation, use and storage, in order to avoid undesired side reactions.
- As for the biosafety of such materials in humans, the biocompatibility of PDA was established based on observations made on cells and animal models (mice or rats). A better understanding of the pharmacokinetics profiles, the behavior towards physiological barriers, and the biodegradation pathways needs to be fulfilled.
Acknowledgments
IZ is thankful to the French Ministry of Research for the financial support of her PhD thesis. The financial supports for PDT and PTT research from the ANR JCJC Grant (Project-ANR-19-CE09-0015) and from the Laboratory of Excellence LERMIT via an ANR grant (ANR-10-LABX-33) under the program of 'Investissements d'avenir' are gratefully acknowledged.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).