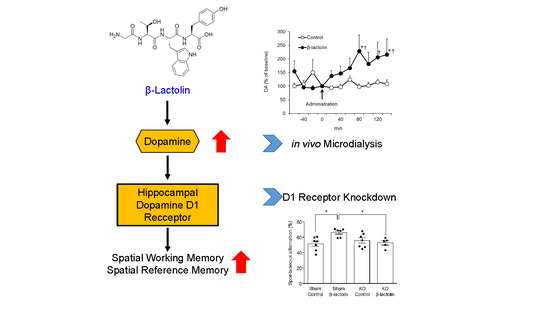
The Lacto-Tetrapeptide Gly–Thr–Trp–Tyr, β-Lactolin, Improves Spatial Memory Functions via Dopamine Release and D1 Receptor Activation in the Hippocampus
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Animals
2.3. In Vivo Microdialysis
2.4. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.5. Adeno-Associated Virus (AAV) Injection
2.6. Y-Maze Test
2.7. Novel Object Recognition Test (NORT)
2.8. Novel Object Location Test (NOLT)
2.9. Dosage Information
2.10. Statistical Analysis
3. Results
3.1. β-Lactolin Increases the Extracellular Concentration of Dopamine in the Hippocampus
3.2. β-Lactolin Improves Spatial Working Memory via the Dopamine D1 Receptor in the Hippocampus
3.3. The Dopamine D1 Receptor in the Hippocampus Is Vital for the Enhancing Effect of β-Lactolin on Spatial Reference Memory
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAV | adeno-associated virus |
| AD | Alzheimer’s disease |
| GTWY | glycine–threonine–tryptophan–tyrosine |
| LTP | long-term synaptic potentiation |
| MAO | monoamine oxidase |
| MWM | Morris water maze |
| WY | tryptophan–tyrosine |
References
- Camfield, D.A.; Owen, L.; Scholey, A.B.; Pipingas, A.; Stough, C. Dairy constituents and neurocognitive health in ageing. Br. J. Nutr. 2011, 106, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crichton, G.E.; Murphy, K.J.; Bryan, J. Dairy intake and cognitive health in middle-aged South Australians. Asia Pac. J. Clin. Nutr. 2010, 19, 161–171. [Google Scholar] [PubMed]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Doi, Y.; Uchida, K.; Shirota, T.; Yonemoto, K.; Kitazono, T.; Kiyohara, Y. Dietary patterns and risk of dementia in an elderly Japanese population: The Hisayama Study. Am. J. Clin. Nutr. 2013, 97, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Kutsukake, T.; Hoshi, A.; Yoshida, A.; Nakayama, H. Identification of a novel dehydroergosterol enhancing microglial anti-inflammatory activity in a dairy product fermented with Penicillium candidum. PLoS ONE 2015, 10, e0116598. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Ozawa, M.; Kutsukake, T.; Sugiyama, S.; Uchida, K.; Yoshida, A.; Nakayama, H. Preventive effects of a fermented dairy product against Alzheimer’s disease and identification of a novel oleamide with enhanced microglial phagocytosis and anti-inflammatory activity. PLoS ONE 2015, 10, e0118512. [Google Scholar] [CrossRef]
- Ano, Y.; Kutsukake, T.; Sasaki, T.; Uchida, S.; Yamada, K.; Kondo, K. Identification of a Novel Peptide from beta-Casein That Enhances Spatial and Object Recognition Memory in Mice. J. Agric. Food Chem. 2019, 67, 8160–8167. [Google Scholar] [CrossRef]
- Ano, Y.; Ayabe, T.; Kutsukake, T.; Ohya, R.; Takaichi, Y.; Uchida, S.; Yamada, K.; Uchida, K.; Takashima, A.; Nakayama, H. Novel lactopeptides in fermented dairy products improve memory function and cognitive decline. Neurobiol. Aging 2018, 72, 23–31. [Google Scholar] [CrossRef]
- Ano, Y.; Ayabe, T.; Ohya, R.; Kondo, K.; Kitaoka, S.; Furuyashiki, T. Tryptophan-Tyrosine Dipeptide, the Core Sequence of beta-Lactolin, Improves Memory by Modulating the Dopamine System. Nutrients 2019, 11, 348. [Google Scholar] [CrossRef]
- Da Silva, W.C.; Kohler, C.C.; Radiske, A.; Cammarota, M. D1/D5 dopamine receptors modulate spatial memory formation. Neurobiol. Learn. Mem. 2012, 97, 271–275. [Google Scholar] [CrossRef] [Green Version]
- De Bundel, D.; Femenia, T.; DuPont, C.M.; Konradsson-Geuken, A.; Feltmann, K.; Schilstrom, B.; Lindskog, M. Hippocampal and prefrontal dopamine D1/5 receptor involvement in the memory-enhancing effect of reboxetine. Int. J. Neuropsychopharmacol. 2013, 16, 2041–2051. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.R.; Sun, N.; Lei, L.; Li, X.Y.; Yao, B.; Sun, K.; Hu, R.; Zhang, X.; Shi, X.D.; Gao, C. L-Stepholidine rescues memory deficit and synaptic plasticity in models of Alzheimer’s disease via activating dopamine D1 receptor/PKA signaling pathway. Cell Death Dis. 2015, 6, e1965. [Google Scholar] [CrossRef] [PubMed]
- Yuan Xiang, P.; Janc, O.; Grochowska, K.M.; Kreutz, M.R.; Reymann, K.G. Dopamine agonists rescue Abeta-induced LTP impairment by Src-family tyrosine kinases. Neurobiol. Aging 2016, 40, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, R.; Taniguchi, M.; Ehrlich, A.T.; Yokogawa, K.; Deguchi, Y.; Cherasse, Y.; Lazarus, M.; Urade, Y.; Ogawa, A.; Kitaoka, S.; et al. Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice. Mol. Psychiatry 2017. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Ohya, R.; Taniguchi, Y.; Shindo, K.; Kondo, K.; Ano, Y. Matured Hop-Derived Bitter Components in Beer Improve Hippocampus-Dependent Memory Through Activation of the Vagus Nerve. Sci. Rep. 2018, 8, 15372. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Ohya, R.; Kondo, K.; Ano, Y. Iso-alpha-acids, bitter components of beer, prevent obesity-induced cognitive decline. Sci. Rep. 2018, 8, 4760. [Google Scholar] [CrossRef]
- Bar-Am, O.; Amit, T.; Kupershmidt, L.; Aluf, Y.; Mechlovich, D.; Kabha, H.; Danovitch, L.; Zurawski, V.R.; Youdim, M.B.; Weinreb, O. Neuroprotective and neurorestorative activities of a novel iron chelator-brain selective monoamine oxidase-A/monoamine oxidase-B inhibitor in animal models of Parkinson’s disease and aging. Neurobiol. Aging 2015, 36, 1529–1542. [Google Scholar] [CrossRef]
- Justo, L.A.; Duran, R.; Alfonso, M.; Fajardo, D.; Faro, L.R.F. Effects and mechanism of action of isatin, a MAO inhibitor, on in vivo striatal dopamine release. Neurochem. Int. 2016, 99, 147–157. [Google Scholar] [CrossRef]
- Schulz, D.; Mirrione, M.M.; Henn, F.A. Cognitive aspects of congenital learned helplessness and its reversal by the monoamine oxidase (MAO)-B inhibitor deprenyl. Neurobiol. Learn. Mem. 2010, 93, 291–301. [Google Scholar] [CrossRef]
- Gangarossa, G.; Longueville, S.; De Bundel, D.; Perroy, J.; Herve, D.; Girault, J.A.; Valjent, E. Characterization of dopamine D1 and D2 receptor-expressing neurons in the mouse hippocampus. Hippocampus 2012, 22, 2199–2207. [Google Scholar] [CrossRef]
- Puighermanal, E.; Cutando, L.; Boubaker-Vitre, J.; Honore, E.; Longueville, S.; Herve, D.; Valjent, E. Anatomical and molecular characterization of dopamine D1 receptor-expressing neurons of the mouse CA1 dorsal hippocampus. Brain Struct. Funct. 2017, 222, 1897–1911. [Google Scholar] [CrossRef]
- Wang, F.; Wan, P.; Wang, W.; Xiao, B.; Jin, H.; Jin, Q. Dopamine in the hippocampal dentate gyrus modulates spatial learning via D1-like receptors. Brain Res. Bull. 2018, 144, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kulla, A.; Manahan-Vaughan, D. Depotentiation in the dentate gyrus of freely moving rats is modulated by D1/D5 dopamine receptors. Cereb. Cortex 2000, 10, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.; Stackman, R.W., Jr. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 2015, 285, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Morici, J.F.; Bekinschtein, P.; Weisstaub, N.V. Medial prefrontal cortex role in recognition memory in rodents. Behav. Brain Res. 2015, 292, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Takuma, K.; Kamei, H.; Ito, Y.; Nakamichi, N.; Ibi, D.; Nakanishi, Y.; Murai, M.; Mizoguchi, H.; Nabeshima, T.; et al. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn. Mem. 2007, 14, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazari-Serenjeh, F.; Rezayof, A.; Zarrindast, M.R. Functional correlation between GABAergic and dopaminergic systems of dorsal hippocampus and ventral tegmental area in passive avoidance learning in rats. Neuroscience 2011, 196, 104–114. [Google Scholar] [CrossRef]
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef]
- Xing, B.; Guo, J.; Meng, X.; Wei, S.G.; Li, S.B. The dopamine D1 but not D3 receptor plays a fundamental role in spatial working memory and BDNF expression in prefrontal cortex of mice. Behav. Brain Res. 2012, 235, 36–41. [Google Scholar] [CrossRef]
- Sarinana, J.; Kitamura, T.; Kunzler, P.; Sultzman, L.; Tonegawa, S. Differential roles of the dopamine 1-class receptors, D1R and D5R, in hippocampal dependent memory. Proc. Natl. Acad. Sci. USA 2014, 111, 8245–8250. [Google Scholar] [CrossRef] [Green Version]
- Kita, M.; Obara, K.; Kondo, S.; Umeda, S.; Ano, Y. Effect of Supplementation of a Whey Peptide Rich in Tryptophan-Tyrosine-Related Peptides on Cognitive Performance in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2018, 10, 899. [Google Scholar] [CrossRef]
- Chowdhury, R.; Guitart-Masip, M.; Bunzeck, N.; Dolan, R.J.; Duzel, E. Dopamine modulates episodic memory persistence in old age. J. Neurosci. 2012, 32, 14193–14204. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Guitart-Masip, M.; Lambert, C.; Dayan, P.; Huys, Q.; Duzel, E.; Dolan, R.J. Dopamine restores reward prediction errors in old age. Nat. Neurosci. 2013, 16, 648–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| Gapdh | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
| Drd1 | AGATGACTCCGAAGGCAGCCTT | GCCATGTAGGTTTTGCCTTGTGC |
| Drd2 | CCTGTCCTTCACCATCTCTTGC | TAGACCAGCAGGGTGACGATGA |
| Drd3 | ACCCTGGATGTCATGATGTG | GGCATGACCACTGCTGTGTA |
| Drd4 | CCTCTCTTTGTCTACTCCGAGGT | GCCATGAGCGTGTCACAG |
| Drd5 | TCCTGGTGTGCTTATGCTTTC | TCAGCTAAGAATCGTTTGGTTTC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayabe, T.; Ano, Y.; Ohya, R.; Kitaoka, S.; Furuyashiki, T. The Lacto-Tetrapeptide Gly–Thr–Trp–Tyr, β-Lactolin, Improves Spatial Memory Functions via Dopamine Release and D1 Receptor Activation in the Hippocampus. Nutrients 2019, 11, 2469. https://doi.org/10.3390/nu11102469
Ayabe T, Ano Y, Ohya R, Kitaoka S, Furuyashiki T. The Lacto-Tetrapeptide Gly–Thr–Trp–Tyr, β-Lactolin, Improves Spatial Memory Functions via Dopamine Release and D1 Receptor Activation in the Hippocampus. Nutrients. 2019; 11(10):2469. https://doi.org/10.3390/nu11102469
Chicago/Turabian StyleAyabe, Tatsuhiro, Yasuhisa Ano, Rena Ohya, Shiho Kitaoka, and Tomoyuki Furuyashiki. 2019. "The Lacto-Tetrapeptide Gly–Thr–Trp–Tyr, β-Lactolin, Improves Spatial Memory Functions via Dopamine Release and D1 Receptor Activation in the Hippocampus" Nutrients 11, no. 10: 2469. https://doi.org/10.3390/nu11102469