Abstract
In the last 25 years microbeam radiation therapy (MRT) has emerged as a promising alternative to conventional radiation therapy at large, third generation synchrotrons. In MRT, a multi-slit collimator modulates a kilovoltage x-ray beam on a micrometer scale, creating peak dose areas with unconventionally high doses of several hundred Grays separated by low dose valley regions, where the dose remains well below the tissue tolerance level. Pre-clinical evidence demonstrates that such beam geometries lead to substantially reduced damage to normal tissue at equal tumour control rates and hence drastically increase the therapeutic window. Although the mechanisms behind MRT are still to be elucidated, previous studies indicate that immune response, tumour microenvironment, and the microvasculature may play a crucial role. Beyond tumour therapy, MRT has also been suggested as a microsurgical tool in neurological disorders and as a primer for drug delivery.
The physical properties of MRT demand innovative medical physics and engineering solutions for safe treatment delivery. This article reviews technical developments in MRT and discusses existing solutions for dosimetric validation, reliable treatment planning and safety. Instrumentation at synchrotron facilities, including beam production, collimators and patient positioning systems, is also discussed. Specific solutions reviewed in this article include: dosimetry techniques that can cope with high spatial resolution, low photon energies and extremely high dose rates of up to 15 000 Gy s−1, dose calculation algorithms—apart from pure Monte Carlo Simulations—to overcome the challenge of small voxel sizes and a wide dynamic dose-range, and the use of dose-enhancing nanoparticles to combat the limited penetrability of a kilovoltage energy spectrum. Finally, concepts for alternative compact microbeam sources are presented, such as inverse Compton scattering set-ups and carbon nanotube x-ray tubes, that may facilitate the transfer of MRT into a hospital-based clinical environment.
Intensive research in recent years has resulted in practical solutions to most of the technical challenges in MRT. Treatment planning, dosimetry and patient safety systems at synchrotrons have matured to a point that first veterinary and clinical studies in MRT are within reach. Should these studies confirm the promising results of pre-clinical studies, the authors are confident that MRT will become an effective new radiotherapy option for certain patients.
Export citation and abstract BibTeX RIS
1. Introduction
Despite the technical and biological advances of modern radiotherapy, there are many types of cancer that have not seen significant improvements in prognosis. For example, paediatric diffuse intrinsic pontine glioma, an aggressive brainstem tumour, has a survival rate of less than 10% at two years following diagnosis (Hargrave et al 2006). Locally advanced pancreatic cancer and glioblastoma multiforme both have survival rates of less than 10% at five years (Stupp et al 2009, Siegel et al 2015). Chondrosarcoma, a notoriously aggressive cancer of cartilaginous cells, is usually resistant to both chemotherapy and conventional radiotherapy, making surgical resection—often amputation—the main effective treatment option (Bovée et al 2005, Cesari et al 2007, Riedel et al 2009). In these scenarios, the intrinsic characteristics of the disease, or the sensitivity of surrounding organs to radiation, hinders any opportunity for lasting disease control.
These dismal outcomes suggest that a paradigm shift could be required to improve prognosis and perhaps provide the possibility of cure. Spatially fractionated radiotherapy using microbeams is a radical departure from the physical properties and radiobiological principles of conventional radiotherapy. The purpose of this review is to explore the physics and technical developments fundamental to the field of microbeam radiotherapy (MRT).
1.1. Paradigms in modern radiation oncology
The broad aim of conventional radiotherapy is to safely deliver the highest possible homogenous dose to the target volume. This objective is primarily achieved through the temporal fractionation of dose and geometric dose-conformity to the target. Linear accelerators are the workhorse of modern radiotherapy clinics globally, facilitating the treatment of a diverse range of tumours in virtually any location in the body. Most linear accelerators generate mega-electron-volt (MeV) x-rays and electrons, operate at a dose rate in the order of 0.1 Gy s−1 and produce homogenous fields of radiation that can be collimated or modulated to optimise the geometry of dose-distributions in tissue.
Early in the 20th century, the first radiotherapy treatments were delivered as a large single fraction and associated with significant morbidity and physical disfigurement. These toxic effects were the backdrop for one of the most fundamental developments in radiation oncology; temporal fractionation. In the early 1920s, Claudius Regaud and his French compatriot Henri Coutard demonstrated that healthy tissue could better tolerate a course of radiotherapy when delivered as a series of several smaller doses over consecutive days, without compromising tumour control (Holsti 1995). Splitting a large radiation dose into multiple smaller fractions capitalises on four of the 'Five Rs of Radiotherapy' Hall and Giaccia (2012). In healthy tissue, there is opportunity for the repair of non-lethal DNA damage and repopulation of the normal cell niche. Simultaneously, the processes of reoxygenation and cell-cycle redistribution increase tumour radio-sensitivity. Temporal fractionation gained acceptance in the wider radiation therapy community by the 1930s and remains a cornerstone of modern radiation oncology.
The therapeutic effect of radiotherapy is also fundamentally maximised by increasing the geometric conformity of dose to the tumour target. Given that healthy tissue surrounding the tumour limits the maximum dose deliverable, the high-dose region is sculpted as closely as possible to the target volume. The target typically includes the gross tumour volume plus an additional margin in normal tissue to account for sub-clinical spread and uncertainties in target position. The majority of technical advancements in modern radiation oncology—intensity modulation (Fraass 1995, Boyer and Cedric 1999), image-guidance (van Herk 2007, Raaymakers et al 2017), motion-management (Lin et al 2013, Zagar et al 2017), particle therapy (Schulz-Ertner et al 2006)—have revolved around improving the conformity of dose to the target, reducing collateral damage to healthy tissue and facilitating dose-escalation.
1.2. Spatially fractionated radiotherapy
The concept of spatial fractionation is built on the dose-volume effect; that the tolerance of normal tissue to radiation increases as the irradiated volume of that specific tissue is reduced (Hopewell et al 1987, Emami et al 1991, Marks et al 2010). Alban Köhler first conceived the idea of applying spatial fractionation to radiotherapy in 1909. Köhler showed that skin toxicity could be reduced using 'grid therapy', where a 3 mm2 grid of woven iron wire was pressed closely to the skin of patients during kilovoltage irradiation (Laissue et al 2012). Today, macroscopic grid therapy using megavoltage x-rays from a linear accelerator is used to de-bulk large and advanced tumours prior to conventional radiation therapy (RT) (Zhang et al 2008), however this technique is not widely used.
Several forms of spatially fractionated radiotherapy, including MRT, are currently in pre-clinical development. While MRT is the focus of this review, there are other noteworthy modalities including minibeam radiotherapy, which utilises arrays of sub-millimetre (0.4–0.7 mm) planar beams of x-rays (Gil et al 2011, Prezado et al 2012b, 2015) or protons (Peucelle et al 2015, Prezado et al 2017b), and microchannel irradiation using arrays of x-ray or proton microbeams (Schültke et al 2013, Girst et al 2015).
1.3. Microbeam radiotherapy
At present, MRT is the most extensively investigated modality that employs spatial dose-fractionation. Microbeams were first used in medicine in the 1960s to understand the effects of cosmic radiation. At that time, Zeman et al (1961) reported that the tolerance of mouse brain tissue to a single beam of deuterons could be increased from 140 Gy to 4000 Gy when reducing the diameter of the beam from 1 mm to 25  m. While the work of Zeman and colleagues exemplified the dose-volume effect, it was not until the late 1980s that true spatial dose-fractionation using microbeams was demonstrated.
m. While the work of Zeman and colleagues exemplified the dose-volume effect, it was not until the late 1980s that true spatial dose-fractionation using microbeams was demonstrated.
MRT was first explored by Daniel Slatkin and colleagues at the Brookhaven National Laboratory in the late 1980s and early 1990s (Slatkin et al 1992, 1995). MRT has been in pre-clinical development at a small number of synchrotrons across the world ever since, including the European Synchrotron Radiation Facility (ESRF) (Grenoble, France), SPring-8 (Hyogo Prefecture, Japan), the Australian Synchrotron (Melbourne, Australia) and the Canadian Light Source (Saskatoon, Canada).
In MRT, spatial dose-fractionation is achieved on a microscopic scale. Fields are characterised by an array of 25–100  m wide, quasi-parallel, micro-planar beams that have a centre-to-centre spacing of 100–400
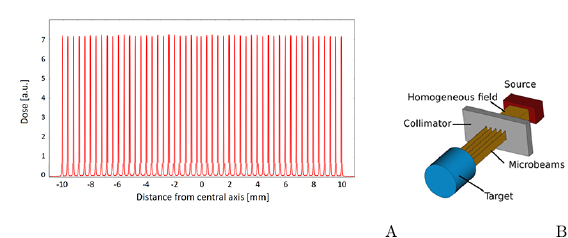
m wide, quasi-parallel, micro-planar beams that have a centre-to-centre spacing of 100–400  m (Bräuer-Krisch et al 2010). This kind of array creates an inhomogeneous, periodically alternating dose profile of 'peaks' and 'valleys' (figure 1). In-beam doses (peaks) can be up to 100 times higher than the dose between the beams (valleys) due to scatter (Blattmann et al 2005). In pre-clinical in vivo studies, peak to valley dose ratios (PVDRs) are more commonly in the range of 20–50 (Serduc et al 2009, Laissue et al 2013, Mukumoto et al 2017, Schültke et al 2018, Smyth et al 2018). The physical characteristics of MRT are discussed more thoroughly in sections 2 and 4.
m (Bräuer-Krisch et al 2010). This kind of array creates an inhomogeneous, periodically alternating dose profile of 'peaks' and 'valleys' (figure 1). In-beam doses (peaks) can be up to 100 times higher than the dose between the beams (valleys) due to scatter (Blattmann et al 2005). In pre-clinical in vivo studies, peak to valley dose ratios (PVDRs) are more commonly in the range of 20–50 (Serduc et al 2009, Laissue et al 2013, Mukumoto et al 2017, Schültke et al 2018, Smyth et al 2018). The physical characteristics of MRT are discussed more thoroughly in sections 2 and 4.
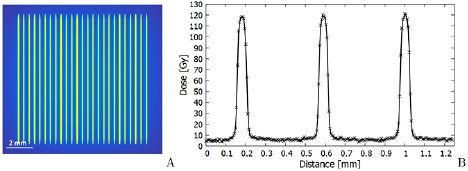
Figure 1. (A): A typical beam profile of microbeams, simulated for a  field in 10 mm depth in water at the imaging and medical beamline of the Australian Synchrotron. The principle of microbeam generation is shown in (B). A collimator shapes microbeams from a homogeneous x-ray beam.
field in 10 mm depth in water at the imaging and medical beamline of the Australian Synchrotron. The principle of microbeam generation is shown in (B). A collimator shapes microbeams from a homogeneous x-ray beam.
Download figure:
Standard image High-resolution imagePeak doses used in pre-clinical MRT experiments usually fall in the range of 100–1000 Gy (Schültke et al 2008, Serduc et al 2008, Laissue et al 2013, Ibahim et al 2014). These extremely high peak doses are tolerated by a range of healthy tissues with minimal structural or physiological deficits (Laissue et al 2007, 2013, Serduc et al 2008, Van Der Sanden et al 2010, Smyth et al 2018). In addition to the remarkable tolerance of normal tissue to peak doses, pre-clinical studies show that MRT can slow tumour growth and even facilitate tumour control despite not irradiating the entire tumour with a uniform field (Laissue et al 1998, Miura et al 2006, Bouchet et al 2016).
While compact MRT delivery systems have also been developed (Hadsell et al 2014, Bartzsch et al 2016) and are reviewed in section 6, the properties of synchrotron radiation are optimal for delivering the peak-valley dose-distribution intrinsic to MRT (Slatkin et al 1992). Firstly, the keV x-ray energy minimises the range of secondary electrons in the valley region, preserving a high PVDR. Secondly, the ultra high dose rate in the range of kGy/s mitigates the effects of physiological tissue motion, including the cardio-synchronous pulsation of blood vessels and respiration. Lastly, minimal beam divergence is required to maintain the array geometry on a microscopic scale. The high dose-rates and peak doses, in combination with microscopic spatial resolution, makes physical methods of dosimetry very demanding. Techniques to facilitate synchrotron-based MRT dosimetry are discussed in section 3.
There are a number of mechanisms that have been proposed for the therapeutic efficacy of MRT. Firstly, normal tissue retains its cellular architecture and the ability to launch a coordinated repair response following MRT while certain tumour tissues (e.g. breast tumour) demonstrate marked cellular migration and reduced proliferative capacity (Crosbie et al 2010). Secondly, MRT exerts differential transcriptomic effects on tumour and normal tissue, with differences in key pathways relating to immunity and inflammation (Bouchet et al 2013b, 2015). The regulation of inflammation and immune response is also different when comparing tissue irradiated using MRT versus conventional RT (Sprung et al 2012, Yang et al 2014, Ibahim et al 2015), which further highlights the potential importance of these pathways to the therapeutic effect of MRT. Thirdly, tumour micro-vasculature has a greater radio-sensitivity to MRT compared to normal brain micro-vasculature (Bouchet et al 2010, 2013a), which has implications for vascular permeability and the delivery of micro-nutrients, cellular mediators of damage repair and immune cell recruitment. Finally, the ultra-high dose-rate of MRT—several hundred (Livingstone et al 2017) to several thousand (Renier et al 2008) Gray per second—may contribute to improved normal tissue sparing via what is now known as the FLASH effect (Favaudon et al 2014).
The degree to which the FLASH effect might contribute to the normal tissue sparing characteristics of MRT has not yet been elucidated. However, as a stand-alone technique, broad-beam FLASH radiotherapy at dose-rates greater than 40 Gy s−1 reduces lung fibrosis (Favaudon et al 2014) and mitigates brain injury (Montay-Gruel et al 2017, 2018) in rodent models compared to irradiation at conventional dose-rates (0.1 Gy s−1). These tissue-sparing phenomena have since been reproduced in large animal models, with pet cats bearing spontaneous facial cancers also experiencing favourable tumour control outcomes following treatment with FLASH radiotherapy (Vozenin et al 2019).
1.4. Potential clinical applications of MRT in medicine
MRT is currently in a pre-clinical phase and to date, no human patients have been treated with MRT. The current challenge is to develop safe protocols that maximally exploit the unique radiobiological properties of MRT. A diverse range of potential clinical applications of MRT have been identified and explored through in vivo studies.
The biological rationale for MRT as a stand-alone, combination, or neoadjuvant treatment has been established in pre-clinical studies. Significant technical developments in the realm of medical physics are required to facilitate future pre-clinical and veterinary studies, and ultimately, the first human trials of MRT. These developments are the focus of this review.
1.4.1. MRT as a boost for conventional radiotherapy.
Schültke et al (2017) propose that MRT could be used as an integrated boost within a conventional radiotherapy regimen. Here, the valley dose would match the daily prescribed conventional radiotherapy dose while the peaks would act as a simultaneous boost to enhance tumour control (Schültke et al 2017). Bouchet et al (2016) provide a rationale for this approach by demonstrating better overall survival, in a rodent glioma model, following MRT compared to broad-beam irradiation when the MRT valley dose was matched to the broad-beam dose.
1.4.2. MRT as a primer for drug delivery
The previous potential applications of MRT largely draw on the normal tissue sparing properties of spatial fractionation, allowing for dose-escalation to the tumour. However, the differential effect of MRT on tumour and normal microvasculature (Bouchet et al 2010) makes MRT a potentially potent primer for drug delivery by inducing a window of enhanced vascular permeability in the tumour (Bouchet et al 2017). Similarly, the immunomodulatory properties of MRT (Bouchet et al 2013b, Yang et al 2014, Brönnimann et al 2016) could be exploited in combination with immunotherapy. Pre-clinical studies have demonstrated the synergistic effect of MRT in combination with a range of drugs (Régnard et al 2008, Bouchet et al 2012) and immunotherapy (Smilowitz et al 2006). However, the optimal dose and timing of MRT in this setting, and the choice of ideal chemo- or immunotherapeutics to test in combination, remains to be determined.
1.4.3. MRT as a micro-surgical tool in neurological disorders
Alongside possible applications in radiation oncology, MRT has the potential to alleviate the symptoms of neurological conditions. In this context, MRT could be used to deliberately transect key neuronal pathways or ablate highly localised regions of the brain in order to modulate or suppress the networks responsible for abnormal movement (Serduc et al 2010b, Romanelli and Bravin 2011). Proof of principle data supporting these neurosurgical applications of MRT exists in pre-clinical models of spinal cord injury (Dilmanian et al 2012) and epilepsy related to the somatosensory cortex (Pouyatos et al 2013). Epilepsy induced by mesial temporal sclerosis may also benefit from this application of MRT (Fardone et al 2018).
2. Technical development and engineering
2.1. Properties of synchrotron radiation
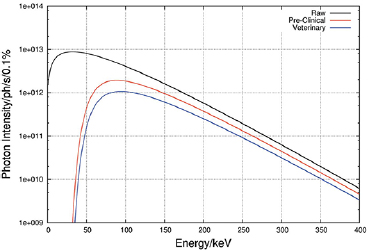
The discovery of synchrotron radiation in 1946 (Elder et al 1947) and the subsequent development of synchrotron radiation research centers around the world have revolutionized x-ray science. Modern synchrotron sources at dedicated storage rings have a brilliance (number of photons/s/mm2/mrad2 within a bandwidth of 0.1%) in the order of 1021 as compared to 107 for conventional x-ray tubes (Kim 1986, Duke 2009, Winick and Doniach 2012). Currently, the most appropriate x-ray source for MRT is a so-called wiggler (as opposed to a bending magnet or an undulator), which provides a continuous high photon flux spectrum and a sufficiently large horizontal radiation fan for the desired size of the radiation field (see figure 2). The main considerations for the optimization of the MRT photon spectrum relate to (i) maximising the photon flux to allow for the required dose rate delivery; (ii) providing the necessary x-ray energy to reach deep-lying targets, and (iii) maximising the peak-to-valley-dose ratio (PVDR). In this context, photon energies below 50 keV are considered not useful; these are therefore filtered out by the insertion of a set of absorption filters (see below).
Figure 2. Typical unfiltered and filtered wiggler spectrum for MRT applications. Example of ID17 at the ESRF. Figure from Crosbie et al (2015) reproduced with permission of the International Union of Crystallography, https://doi.org/10.1107/S1600577515008115.
Download figure:
Standard image High-resolution imageOn the other hand, extensive MC calculations of an array of micro-beams revealed that a mean photon energy around 100–150 keV gives the best compromise between a well-defined peak dose profile with a sharp fall-off towards the valley dose region (Spiga et al 2007a, Donzelli et al 2018) (see section 4).
2.2. General beamline lay-out
The typical beamline lay-out for MRT has been described in detail in the past (Martínez-Rovira et al 2012b, Cornelius et al 2014, Wysokinski et al 2015, Stevenson et al 2017). Standard components comprise horizontal and vertical slits to reduce the heatload and to define the broad and MRT beam dimensions. A filter train eliminates the low-energy part of the spectrum. To monitor the beam stability, ionization chambers (IC) or a Compton chamber beam monitor are installed. Preclinical studies with small and large animals require slightly different conditions in terms of spectral filtering, intensity, and dose monitoring (Martínez-Rovira et al 2012b, Cornelius et al 2014, Crosbie et al 2015).
2.3. Fast shutters
The radiation dose delivered to the target, needs to be accurately controlled in order to prevent unnecessary damage to surrounding, normal tissues. Implementation of a dedicated photon shutter system, combined with a reliable, redundant interlock system, prevents any excessive dose delivery.
The system, implemented on the biomedical beamline ID17 at the ESRF, consists of a standard photon absorber, followed down-stream by a fast shutter device (Renier et al 2002). The measured error in the exposure time of the fast shutter amounts to  0.5 ms, which results in a 1% error for typical exposure times of 50 ms.
0.5 ms, which results in a 1% error for typical exposure times of 50 ms.
2.4. Multislit collimator
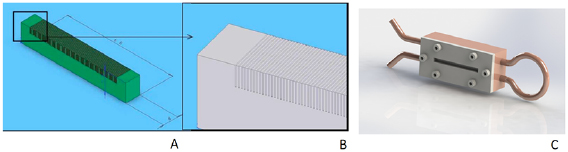
One of the key elements in MRT is the multislit collimator (MSC) since the mechanical regularity of such devices is the most important property required to produce an array of identical microbeams. Following first designs (Archer 1998, Bräuer-Krisch et al 2005a), the currently most utilized MSC is a single slit device composed of 8 mm thick blocks of tungsten carbide (WC), presenting 125, three mm high, and 50  m wide equidistant slits to the incoming seamless x-ray beam, regularly repeated with a uniform pitch of 400
m wide equidistant slits to the incoming seamless x-ray beam, regularly repeated with a uniform pitch of 400  m (Bräuer-Krisch et al 2009). The MSC chamber is mounted on a rotational stage, equipped with a motorized translation in the vertical direction and in the horizontal direction perpendicular to the x-ray beam, for rapid alignment. Schematic drawings of the MSC and its assembly are shown in figure 3.
m (Bräuer-Krisch et al 2009). The MSC chamber is mounted on a rotational stage, equipped with a motorized translation in the vertical direction and in the horizontal direction perpendicular to the x-ray beam, for rapid alignment. Schematic drawings of the MSC and its assembly are shown in figure 3.
Figure 3. (A): Schematic drawing of a MSC with 100  m slit width and a pitch of 400
m slit width and a pitch of 400  m and (B): zoom of the assembly; (C): MSC embedded inside the copper block with its pipes for water cooling.
m and (B): zoom of the assembly; (C): MSC embedded inside the copper block with its pipes for water cooling.
Download figure:
Standard image High-resolution imageAn extensive characterization of the MSC led to 404  m (SD 10
m (SD 10  m) for the regularity of the slit spacing, and 51.8
m) for the regularity of the slit spacing, and 51.8  m (SD 1.1
m (SD 1.1  m) for the slit width.
m) for the slit width.
2.5. Sample goniometer and patient positioning system
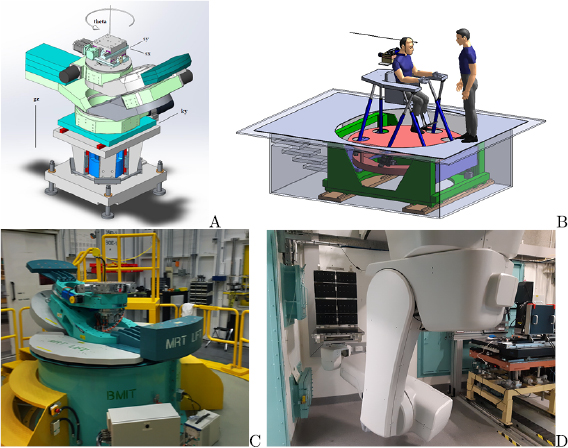
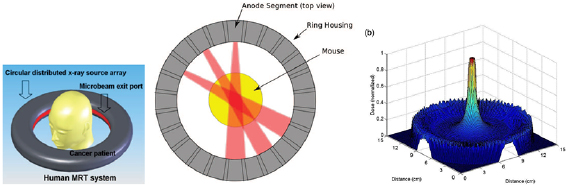
Sample and patient positioning systems for MRT are different from positioning systems used in conventional RT due to the use of a fixed horizontal beam rather than a rotating gantry. In addition, the limited beam height requires the vertical translation of a target through the beam during irradiation. At the ESRF a kappa-type goniometer manufactured by Huber (Germany) is installed (Bräuer-Krisch et al 2005a, Bräuer-Krisch et al 2013) on top of a vertical translational stage (see figure 4(A). Its loading capacity is 35 kg, and the z-stage allows for a total vertical movement of 150 mm at a maximum linear speed of 150 mm s−1. The accuracy in velocity of the translational stage allows for a dose delivery with an accuracy of 5%. In view of the upcoming human clinical trials, a conceptual design study was performed for a patient positioning system (see figure 4(B)). At the BMIT beamline of the Canadian Light Source (CLS) in Saskatoon, Canada, a large animal positioning system (LAPS) is installed capable of holding samples up to 907 kg (Wysokinski et al 2015). The LAPS can move at vertical velocities of up to 200 mm s−1 at 1% accuracy and a spatial accuracy of 100  m. On top of the LAPS a kappa-type goniometer with a loading capacity of up to 120 kg can be installed for 3-axis sample positioning (Wysokinski et al 2013) (see figure 4(C)).
m. On top of the LAPS a kappa-type goniometer with a loading capacity of up to 120 kg can be installed for 3-axis sample positioning (Wysokinski et al 2013) (see figure 4(C)).
Figure 4. Current MRT kappa type goniometer in the MRT experimental hutch for preclinical research in MRT (A) and design of the future patient positioning and irradiation system for MRT clinical trials (B) at the ESRF. A stage for horse MRT trials at the Canadian Lightsource (C). A robotic patient positioning system (PPS) and a large animal positioning system (LAPS) has been installed at the Australian Synchrotron (D).
Download figure:
Standard image High-resolution imageMore recently, the IMBL at the Australian Synchrotron have installed two robotic positioning devices; a so-called large animal positioning system (LAPS) and a Patient Positioning System (PPS) (see figure 4(D)). These robots are located in the long beamline known as Hutch 3B where phase contrast radiography, tomography, and some radiotherapy experiments take place. There are also plans to install another robotic patient positioning device on the near beamline (Hutch 2B) in the future. These robots are similar in scale to the positioning systems used in fixed-beam proton therapy facilities. Whilst these robotic devices are primarily used to image large animals and humans, they can also be used to translate patients vertically through a therapeutic beam. Commissioning work is taking place in 2019 to verify and validate the use of the LAPS and PPS for veterinary trials of synchrotron radiotherapy.
An irradiation sequence starts with the prepositioning of the target, and taking into consideration location marks obtained during prior imaging sessions. By means of the z-stage, the target is driven to the start position, and when the irradiation scan is triggered, the target accelerates vertically to reach the steady speed as chosen by the operator. The fast shutter opens and closes precisely at pre-determined positions depending on the volume to be treated. The target then decelerates and stops, and returns to its start position. The target may be re-oriented in another direction and a further irradiation sequence may start.
2.6. Image-guidance
Image-guidance is a well established technique in conventional RT to increase the spatial precision of a RT treatment. Various imaging techniques (fan and cone beam CT, x-ray projection imaging, MRI) can be used to verify the patient anatomy for planning and the patient set-up prior to and during the treatment.
The special circumstances at the currently available radiation sources for MRT have lead to the development of specialised techniques and protocols for image-guidance adapted to the fixed horizontal beam geometry at synchrotron facilities.
The first image-guided MRT experiment was reported in Serduc et al (2010a) by Serduc et al (2010a) at the ESRF beamline ID17. Their image-guidance protocol used the same radiation source for rat imaging and irradiation. To reduce the x-ray beam intensity and energy, the wiggler gap was opened and additional absorbers were placed in the beam for imaging. The rat imaging was carried out step by step acquiring 1 mm high frames while the animal was translated gradually upwards. A graphical user interface (GUI) displayed the full x-ray image and assisted in the positioning of the animal in the beam using bone structures of the skull as reference points for atlas-based alignment. The rotation was not corrected in this protocol which was used for radiosurgery of somatosensory cortex and thalamus of GAERS rats to investigate the effect of microbeams on epileptic seizures. The same protocol was successfully applied by others, e.g. Romanelli et al (2013).
Zhang et al (2014) used a combination of x-ray projection imaging and pre-irradiation MRI for tumour-bearing rats. They located the tumour on an MRI-image and performed an image-registration of sagittal MRI planes with projection images taken of the animal mounted on the irradiation stage of a carbon nano tube (CNT) based irradiator (Hadsell et al 2013). The applied re-positioning consisted of a translation of the animal in the image plane without any correction for the rotation.
The protocol developed by Nemoz et al (2016) at the ESRF was the first to acquire a full tomographic scan of the region of interest of a rat while the animal is immobilized on the treatment stage. Using a pink synchrotron radiation beam as described in Serduc et al (2010a), a CT of the animal head was acquired in 5 slices of 2 mm height each with a pixel size of 100  m. Iodine as contrast agent was used to enhance tumour contrast on the CT images and the imaging spectrum was adjusted to match the iodine K-edge at 33 keV.
m. Iodine as contrast agent was used to enhance tumour contrast on the CT images and the imaging spectrum was adjusted to match the iodine K-edge at 33 keV.
At the Australian Synchrotron (Pelliccia et al 2016b) developed a small animal image-guidance protocol which employs monochromatic synchrotron radiation for sample imaging. The use of a double-crystal Laue monochromator introduced a 20 mm shift between treatment and imaging beam which required vertical translation of the target in between the two procedures.
The sample imaging was done during a continuous vertical translation through the laminar x-ray beam, while a silicon detector acquired a series of images which were then tiled together to a full-field representation of the sample. To gather additional information on the sample, a CT slice of the plane selected on the projection image can be taken, similar to the procedure of Nemoz et al (2016). The image quality can be improved by relying on phase contrast images (Pelliccia et al 2016a). In addition the recent installation of an independent, external x-ray tube for full-field imaging at the Australian Synchrotron (Livingstone et al 2017), which is orientated perpendicularly to the synchrotron beam, may accelerate the procedure and overcome the impractical translation of the target between imaging and treatment.
The first alignment protocol for large animals was developed for ID17 at the ESRF by Donzelli etal (2016). The protocol was based on individual treatment planning where the target and the beam directions were defined on a CT image with the help of a commercial treatment planning platform. The alignment of the target used fiducial markers as reference points, which were placed on the animal during CT-imaging. Before the treatment, x-ray projection images of the animal on the treatment stage were acquired from different angles. With the aid of manually identified fiducial markers in these projection images, an algorithm calculated the correct translation in 3 dimensions and rotation about 3 axes to be applied to have the beam orientation as defined in the treatment plan. This protocol was used successfully to irradiate small pigs at ID17 in February 2017.
2.7. Organ motion
Organ motion during irradiation can have a serious impact on microbeam dose distributions. The impact of dose blurring has been assessed with MC studies by Donzelli (2018) and Manchado de Sola et al (2018). Donzelli et al (2016) investigated the effect of cardiovascular brain motion during MRT treatment and came to the conclusion that dose rates of 12.3 kGy s−1 are necessary to ensure steep dose penumbras. Such high dose rates currently force any clinical applications to be carried out at large synchrotron facilities. Beyond blurring, organ motion is also hazard when aligning microbeam arrays applied from different directions. In preclinical studies so called interspersed geometries have been created to form almost homogeneous doses in the tumour target (Bräuer-Krisch et al 2005b). It is questionable whether such beam geometry can be reliably applied in clinical applications.
Organ motion introduces considerable risk of misalignment, particularly for interlaced microbeam patterns. Donzelli et al (2019) introduced a concept called spiralMRT, a geometry offering similar spatial fractionation properties as interlaced MRT, while being less vulnerable to target positioning uncertainties. The dose distributions achievable with spiralMRT in a simplified human head geometry were calculated with Monte Carlo simulations based on Geant4 and the dependence of the result on the microbeam pitch, total radiation field size, and photon energy were analysed. A comparison with interlaced MRT and conventional MeV tomotherapy was carried out.
SpiralMRT delivers homogeneous dose distributions to the target, while using spatially fractionated entrance beams. The valley dose of spiralMRT entrance beams is by up to 40% lower than the corresponding tomotherapy dose. SpiralMRT thus offers to be a promising approach to delivering homogeneous dose distributions with spatially fractionated entrance beams, possibly decreasing normal tissue side effects in hypofractionated radiation therapy.
3. Dosimetry
Experimental dosimetry is an essential ingredient, together with state-of-the-art dose calculations, for the development and validation of the TPS. The main challenges in MRT dosimetry are on one hand to determine the very high dose rates in the homogeneous field on an absolute scale, taking into account the necessary corrections to be applied for the typical spectrum from a synchrotron radiation x-ray source, and on the other hand the dose measurement of an array of x-ray micro-beams, modulated on a micrometer scale.
Regarding absolute dose determination in a homogeneous field, ion chambers (section 3.1) are the accepted primary standard, while Alanine-based dosimetry (section 3.2) has obtained increased attention over the past years. This reference dosimetry is performed prior to the spatial fractionation of the x-ray beam, and Monte Carlo methods are used to convert the reference dose to the dose within the micro-beam field.
For micro-scale dosimetry (MSD), i.e. the determination of the absorbed dose with micrometer spatial resolution, commercially available Gafchromic® films are widely utilised, though there are certain limitations as will be detailed in section 3.3. This research has triggered several other developments in experimental MSD. During the last decade several detectors were tested for potential applications in MRT: samarium doped glasses (section 3.4), MOSFET edge-on and silicon strip detectors (section 3.5), high-resolution thermoluminescent dosimeter (TLD, section 3.6), polymer gels (Maryanski et al 1993, De Deene et al 2002, Bayreder et al 2006), Optical Computed Tomography (CT) using a radiochromic plastic named PRESAGE (Doran et al 2010), Fluorescent Nuclear Track Detectors (FNTD) (section 3.7), and optical fiber dosimetry (section 3.8). Most recently, the emergence of commercial, and clinically traceable diamond detectors with micron-scale spatial resolution have appeared on the market and are emerging as potential candidates for MRT dosimetry (section 3.9). Table 1 provides an overview of tested detector systems.
Table 1. Comparison of different high resolution dosimeters tested in MRT.
| Detector | Operating range | Resolution | Dose rate dependence | Energy dependence | Measurement type |
|---|---|---|---|---|---|
| Radiochromic films | 0.1 Gy–400 Gy | 5  m m |
None | Nearly tissue equivalent | Off-line, 2D |
| Sm doped glasses | 1 Gy–1000 Gy | <1  m m |
None | Medium | Off-line, 2D |
| MOSFET detectors | 0.01 Gy–100 Gy | <1  m m |
None | Strong | On-line, 2D |
| Silicon strip | Up to 100kGy | 10 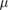 m m |
Unknown | Strong | On-line, 1D |
| TLDs | 0.01 Gy–500 Gy | 5 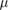 m m |
Unknown | Strong | Off-line, 1D |
| Polymer gels | 0.01 Gy–100 Gy | 100 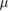 m m |
Strong above 5Gy min−1 | Low | Off-line, 3D |
| PRESAGE | 10 Gy–500 Gy | 20 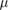 m m |
Unknown | Unknown | Off-line, 3D |
| FNTD | 0.005 Gy–50 Gy | 0.6 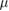 m m |
None | Strong | Off-line, 2D |
| Optical fibre | Measures dose rate >10 Gy s−1 | 10 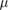 m m |
None | Medium | On-line, 0D |
| Diamond detector | Measures dose rate | 1 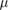 m m |
Low | Low | On-line, 0D |
All of the above dosimetry technologies have specific strengths and weaknesses for the very demanding MRT dosimetry requirements. The Gafchromic® films, silicon detectors, TLDs, FNTDs and diamond detectors seem currently the most adequate and practical dosimeters. All are described in some detail below. Important for the application of microbeams is the determination of output or scatter factors that relate the dose rate in the homogeneous radiation field with the dose rate in the microbeam peaks. These factors are either determined by dosimetry or with Monte Carlo simulations and are a prerequisite for the precise dosage of MRT.
3.1. Ion chambers
Ionization chambers are the dosimetry standard tool in RT protocols for absolute dosimetry (Ma et al 2001, Siegbahn et al 2005). The validation of a treatment planning system (TPS) is usually done with ionization chamber measurements in a liquid water or solid water phantom. A protocol for absolute dose measurements was put in place for MRT preclinical work (Fournier et al 2016). It is based on the International Atomic Energy Agency's TRS 398 absorbed dose-to-water protocol (Andreo et al 2000, Fournier et al 2016).
Reference dosimetry was performed with the PinPoint 31014 IC (sensitive volume of 0.015 cm3) from PTW for a homogeneous field of  cm size and at a depth of 2 cm in a water tank.
cm size and at a depth of 2 cm in a water tank.
The absolute dose in water under reference conditions is given by Andreo et al (2000):

where MQ is the raw reading from the IC corrected for the influence of the temperature and pressure, the polarization between the IC electrodes, the calibration of the electrometer and the ion recombination. Pressure and temperature have to be measured during dosimetry measurement.  is the calibration factor for the beam quality Q0 and
is the calibration factor for the beam quality Q0 and  a factor that corrects for the difference between the beam quality Q0 used for the calibration and the beam quality Q under which the measurements are performed.
a factor that corrects for the difference between the beam quality Q0 used for the calibration and the beam quality Q under which the measurements are performed.
3.2. Alanine dosimeters
Alanine, an amino acid (2-Aminopropanoic acid) is the sensitive material in alanine dosimeters. There are several types of alanine: L or S (+)-alanine, D or R (−)-alanine, beta-alanine etc, but for dosimetry  -Alanine is used (
-Alanine is used ( ). A stable alkyl free radical
). A stable alkyl free radical  is produced upon irradiation. The exposure of an amino acid to ionizing radiation causes the production of radicals of which the number of unpaired electrons is proportional to the absorbed dose over a wide dose range and can be measured by Electron Spin Resonance (ESR) spectroscopy (Poole 1996, Schweiger and Jeschke 2001). The measured signal is linear from approximately 2 Gy–200kGy. Though Alanine dosimetry is not recognised as a primary RT standard, it is widely accepted as secondary standard for absolute dose measurements (Anton 2005, Anton and Lelie 2009). A recent comparative study between PinPoint IC and alanine dosimetry for homogeneous fields revealed an overall agreement between the two methods for a delivered dose between 50 and 5000 Gy of better than 0.7%. The overall uncertainties of the alanine dose measurements were 3.08% and 2.80% at
is produced upon irradiation. The exposure of an amino acid to ionizing radiation causes the production of radicals of which the number of unpaired electrons is proportional to the absorbed dose over a wide dose range and can be measured by Electron Spin Resonance (ESR) spectroscopy (Poole 1996, Schweiger and Jeschke 2001). The measured signal is linear from approximately 2 Gy–200kGy. Though Alanine dosimetry is not recognised as a primary RT standard, it is widely accepted as secondary standard for absolute dose measurements (Anton 2005, Anton and Lelie 2009). A recent comparative study between PinPoint IC and alanine dosimetry for homogeneous fields revealed an overall agreement between the two methods for a delivered dose between 50 and 5000 Gy of better than 0.7%. The overall uncertainties of the alanine dose measurements were 3.08% and 2.80% at  for alanine analysis with an EMX EPR spectrometer and an e-scan benchtop spectrometer, respectively (Soliman et al 2019).
for alanine analysis with an EMX EPR spectrometer and an e-scan benchtop spectrometer, respectively (Soliman et al 2019).
3.3. Radiochromic film dosimetry
Radiochromic films are a common tool in modern radiation therapy dosimetry. Using films, it is possible to measure 2-dimensional dose distributions with sub-millimetric resolution, a fundamental part of treatment plan verification.
The core of the radiochromic dosimeter is a crystalline polyacetylene material responsible for a dose dependent change in optical density. Radiochromic films do not require any chemical treatment, have a weak energy dependence from keV to MeV energies, are dose rate independent and provide spatial resolution between 5 and 25  m depending on the film type. Film dosimetry is a relative dosimetry method and films are usually calibrated with ionisation chambers under reference conditions.
m depending on the film type. Film dosimetry is a relative dosimetry method and films are usually calibrated with ionisation chambers under reference conditions.
In conventional RT, the dose variation of the radiation field occurs on a millimetric scale and the film analysis can be performed with a flatbed scanner, providing the required sub-millimetric resolution. The analysis of film irradiated with 50  m wide microbeams requires a more powerful instrument. Protocols for radiochromic film read-out at the micrometer scale initially used microdensitometers (Crosbie et al 2008, Martínez-Rovira et al 2012b, Bräuer-Krisch et al 2015). The use of a microscope was suggested in 2009 by Nariyama et al (2009) and since then, developed protocols use an inverted optical microscope (Bartzsch et al 2015, Pellicioli et al 2019).
m wide microbeams requires a more powerful instrument. Protocols for radiochromic film read-out at the micrometer scale initially used microdensitometers (Crosbie et al 2008, Martínez-Rovira et al 2012b, Bräuer-Krisch et al 2015). The use of a microscope was suggested in 2009 by Nariyama et al (2009) and since then, developed protocols use an inverted optical microscope (Bartzsch et al 2015, Pellicioli et al 2019).
Microscopes equipped with motorized stages able to move with sub-micrometric precision combined with a charge-coupled device (CCD) camera, allow the acquisition of film areas up to 10 cm2 in a few minutes with micrometric resolution and the evaluation of unusual field configurations such as pencil beams (Schültke et al 2013) or phantoms under motion conditions. Dedicated image processing protocols are being developed to correct the acquired digital images for noise and film inhomogeneities at the micrometric scale (Bartzsch et al 2015, Pellicioli et al 2019). An example of a digitalized film image is reported in figure 5. From the film analysis, dose profiles are obtained with a reproducibility of 1% and read-out uncertainties of less than 5%.
Figure 5. Digital image acquired with an optical inverted microscope of a HD-V2 Gafchromic® film irradiated with a 10 mm  10 mm field of microbeams (A). Dose profile of 50
10 mm field of microbeams (A). Dose profile of 50  m wide and 400
m wide and 400  m pitch microbeams (B).
m pitch microbeams (B).
Download figure:
Standard image High-resolution image3.4. Samarium doped glasses
Another optical method with a resolution of better than 1  m are glasses doped with certain rare earth atoms (Okada et al 2011). Especially the conversion of Sm3+ to Sm2+ has been studied for x-ray dosimetry, since the emission bands in the red part of the optical spectrum are well separated and changes in the fluorescence emission are easily accessible. The spatial variation of radiation dose on the micrometre scale can be read-out using confocal fluorescence microscopy. The detectors are reusable via reconversion of Sm2+ to Sm3+ by thermal annealing (Vahedi et al 2012) or exposure to UV-light (Okada et al 2014). Importantly, the dosimeters show a linear response in a wide dose range from cGy up to 150 Gy and are usable as non-linear dosimeters up to several thousand Gy (Vahedi et al 2012, Okada et al 2014).
m are glasses doped with certain rare earth atoms (Okada et al 2011). Especially the conversion of Sm3+ to Sm2+ has been studied for x-ray dosimetry, since the emission bands in the red part of the optical spectrum are well separated and changes in the fluorescence emission are easily accessible. The spatial variation of radiation dose on the micrometre scale can be read-out using confocal fluorescence microscopy. The detectors are reusable via reconversion of Sm2+ to Sm3+ by thermal annealing (Vahedi et al 2012) or exposure to UV-light (Okada et al 2014). Importantly, the dosimeters show a linear response in a wide dose range from cGy up to 150 Gy and are usable as non-linear dosimeters up to several thousand Gy (Vahedi et al 2012, Okada et al 2014).
3.5. Silicon detectors
Radiation dosimetry in conformal radiotherapy using silicon diodes is well documented in the literature and accepted in the clinical medical physics field. The physical parameters of the MRT beams, however, make it impossible to use such commercial silicon diodes for dosimetry. MOSFET detectors provide a high spatial resolution because of their very small radiation sensitive area defined by the thickness of the MOSFET gate oxide layer. Edge-on MOSFET dosimetry is a technique whereby a MOSFET radiation detector is positioned such that its smallest dimension is normal to the incident beam (Rosenfeld et al 2001).
At the ESRF profiles of a 30  m wide microbeam were successfully acquired using the detector (Rosenfeld et al 2001). The MOSFET detector was also used for measuring the peak and valley doses (Bräuer-Krisch et al 2003). In 2009, the study carried out by Siegbahn et al (2009) reported PVDRs obtained by MC simulations up to 50% higher than the experimental PVDR measured with a MOSFET detector. These discrepancies were mainly attributed to the energy dependence of the detector assuming that there was a significant difference between the photon spectrum in the MRT peak compared to the valley. The MOSFET energy dependence was also highlighted in various articles (Kron et al 1998, De Felici et al 2005, Cheung et al 2009).
m wide microbeam were successfully acquired using the detector (Rosenfeld et al 2001). The MOSFET detector was also used for measuring the peak and valley doses (Bräuer-Krisch et al 2003). In 2009, the study carried out by Siegbahn et al (2009) reported PVDRs obtained by MC simulations up to 50% higher than the experimental PVDR measured with a MOSFET detector. These discrepancies were mainly attributed to the energy dependence of the detector assuming that there was a significant difference between the photon spectrum in the MRT peak compared to the valley. The MOSFET energy dependence was also highlighted in various articles (Kron et al 1998, De Felici et al 2005, Cheung et al 2009).
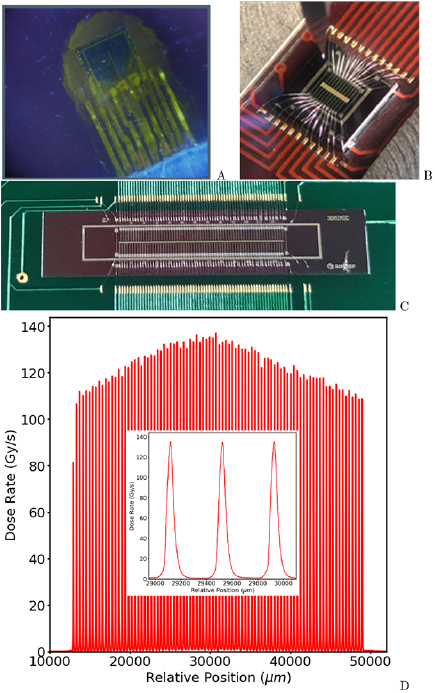
A silicon single-strip detector (SSD) and associated readout electronics have been developed to allow for very high spatial resolution measurements of the instantaneous dose rate at the detector's position in a phantom (Lerch et al 2009, 2011, Petasecca et al 2011) (see figure 6). The SSD response can then be integrated to deduce the total absorbed dose. The dynamic range of the readout system is over five orders of magnitude, which is ideal for MRT dosimetry.
Figure 6. Generations of detectors developed for MRT dosimetry and QA. (A): Single silicon strip detector (SSD); (B): multi-strip silicon detector array (20 strips); (C): back-etched transmission silicon detector array (256 strip) for MRT QA; (D): typical 35 mm wide MRT treatment field map measured at the Australian Synchrotron by the SSD with a zoom of 3 microbeams overlayed.
Download figure:
Standard image High-resolution imageThe SSD design is also suitable for MRT dosimetry where the intrinsic beam height is usually 500  m and microbeam width is typically 50
m and microbeam width is typically 50  m. The single strip active pad area has dimensions of 250
m. The single strip active pad area has dimensions of 250  m to 900
m to 900  m long and 10
m long and 10  m wide. The SSD is fabricated using ion implantation production techniques. The active layer is a 100
m wide. The SSD is fabricated using ion implantation production techniques. The active layer is a 100  cm p-type epitaxial layer that is 50
cm p-type epitaxial layer that is 50  m thick and grown on top of the 370
m thick and grown on top of the 370  m thick silicon substrate of resistivity (0.001
m thick silicon substrate of resistivity (0.001  cm) (Lerch et al 2011, Petasecca et al 2011).
cm) (Lerch et al 2011, Petasecca et al 2011).
In recent applications the SSD has been operated in passive mode where the spatial resolution is close to 20  m in face-on mode. For the read out, a standard clinical electrometer can also be used. In this way, measurements can be more directly compared with other high resolution and clinically relevant dosimeters, under similar irradiation conditions (e.g. ionization chambers in broad beam conditions and other solid state detectors in microbeam conditions). Similar to the MOSFET devices, the best spatial resolution of the SSD of 5
m in face-on mode. For the read out, a standard clinical electrometer can also be used. In this way, measurements can be more directly compared with other high resolution and clinically relevant dosimeters, under similar irradiation conditions (e.g. ionization chambers in broad beam conditions and other solid state detectors in microbeam conditions). Similar to the MOSFET devices, the best spatial resolution of the SSD of 5  m is achievable when it is operated in passive mode, edge-on configuration and in good alignment with the microbeams (Fournier et al 2016, 2017). The combination of the SSD with the fast readout capability of the X-Tream dosimetry system and wide dynamic range allows for precise assessment of the MRT multislit collimator alignment to ensure the accurate reproducibility of the MRT irradiation field and associated dose delivery (see figure 6) (Davis et al 2018).
m is achievable when it is operated in passive mode, edge-on configuration and in good alignment with the microbeams (Fournier et al 2016, 2017). The combination of the SSD with the fast readout capability of the X-Tream dosimetry system and wide dynamic range allows for precise assessment of the MRT multislit collimator alignment to ensure the accurate reproducibility of the MRT irradiation field and associated dose delivery (see figure 6) (Davis et al 2018).
3.6. Thermoluminescence dosimetry
A two-dimensional (2D) thermoluminescence (TL) dosimetry system consisting of  (MCP-N)-based TL foils and a TLD reader equipped with a CCD camera and the large size (72 mm in diameter) planchete heater was developed at the Institute of Nuclear Physics to perform high resolution dosimetry. TLDs have been used for MRT dosimetry; measured dose distributions were compared with Monte Carlo simulations. Measurments confirmed the findings obtained with Gafchromic films, particularly of a measured valley dose of 10%–40% higher than the Mont Carlo predicted dose (Ptaszkiewicza et al 2008).
(MCP-N)-based TL foils and a TLD reader equipped with a CCD camera and the large size (72 mm in diameter) planchete heater was developed at the Institute of Nuclear Physics to perform high resolution dosimetry. TLDs have been used for MRT dosimetry; measured dose distributions were compared with Monte Carlo simulations. Measurments confirmed the findings obtained with Gafchromic films, particularly of a measured valley dose of 10%–40% higher than the Mont Carlo predicted dose (Ptaszkiewicza et al 2008).
3.7. Fluorescent nuclear track detectors
Fluorescent nuclear track detectors (FNTD) are a new type of luminescent detectors for different applications in radiation dosimetry. They were originally developed for neutron and heavy charge particle dosimetry (Akselrod et al 2006) and combine the advantages of solid state track detectors and optical measurements without the need for long chemical etching. The detectors are made of fluorescent aluminum oxide single crystals (sapphire) doped with carbon and magnesium ( ). The tracks of secondary electrons generated by the MRT beams in the single crystal aluminum oxide detector are imaged using a high resolution readout system based on confocal laser scanning fluorescence microscopy (Akselrod et al 2006).
). The tracks of secondary electrons generated by the MRT beams in the single crystal aluminum oxide detector are imaged using a high resolution readout system based on confocal laser scanning fluorescence microscopy (Akselrod et al 2006).
FNTDs were optimized for imaging applications over 4 orders of magnitude of photon doses (Sykora and Akselrod 2010) ranging from 5 mGy to 50 Gy and extremely high spatial resolution of 0.6  m. High spatial resolution and wide dynamic range of dose measurements make FNTD technology very attractive for MRT quality assurance application with a large PVDR (Bartz et al 2011). FNTD is a passive integrating type of detector that does not require wires, electronics or batteries during irradiation. This detector is immune to electromagnetic interference and can measure doses at very high dose rate; it was successfully tested at 108 Gy s−1. FNTD detectors provide extremely good temperature and environmental stability, no light sensitivity, thermal fading or signal build-up. FNTD imaging plates are reusable after thermal annealing or optical bleaching. The most recent results obtained for MRT are summarized in Bartz et al (2011).
m. High spatial resolution and wide dynamic range of dose measurements make FNTD technology very attractive for MRT quality assurance application with a large PVDR (Bartz et al 2011). FNTD is a passive integrating type of detector that does not require wires, electronics or batteries during irradiation. This detector is immune to electromagnetic interference and can measure doses at very high dose rate; it was successfully tested at 108 Gy s−1. FNTD detectors provide extremely good temperature and environmental stability, no light sensitivity, thermal fading or signal build-up. FNTD imaging plates are reusable after thermal annealing or optical bleaching. The most recent results obtained for MRT are summarized in Bartz et al (2011).
3.8. Fiber optical dosimeters
Fiber Optic Dosimeters (FODs) have a significant advantage over many dosimeters developed for MRT in that they are made of plastic scintillators. In an x-ray radiation field environment their water equivalence makes them excellent candidates for MRT and worthy of research and development. FODs typically find use in applications where high spatial resolution (<500  m) is not essential since machining to very small thicknesses is very challenging and plastic scintillators have a low light yield (typically tens of thousands of photons per MeV of energy deposited). The synchrotron light source used in MRT can easily provide the necessary x-ray photon flux to facilitate a measurable response in FODs. However, Cherenkov radiation generation, radioluminescence, radiation hardness and dose rate dependence of FODs requires careful consideration if they are to be used regularly for MRT dosimetry.
m) is not essential since machining to very small thicknesses is very challenging and plastic scintillators have a low light yield (typically tens of thousands of photons per MeV of energy deposited). The synchrotron light source used in MRT can easily provide the necessary x-ray photon flux to facilitate a measurable response in FODs. However, Cherenkov radiation generation, radioluminescence, radiation hardness and dose rate dependence of FODs requires careful consideration if they are to be used regularly for MRT dosimetry.
Optical detectors have been applied to imaging microbeam x-rays in the past (Okada et al 2011, Belley et al 2015), however, they have not been used at highly brilliant synchrotron light sources. Archer et al (2017, 2018) have demonstrated a FOD probe development technique with improved spatial resolution using scintillators and have tested them in an MRT synchrotron x-ray beam, delivering very high dose rates. The scintillator thickness defines the one-dimensional spatial resolution of the FOD probe in the axial direction if it is operated in edge-on mode with respect to the direction and plane of the microbeams. The FOD length is 1 mm in the radial direction as determined by the optical fiber core diameter. The scintillation light generated in the plastic scintillator is transported along an optical fiber to a photomultiplier tube or silicon photomultiplier.
The most recently developed FOD has a one-dimensional spatial resolution of 10  m. The detector is able to resolve the individual microbeams, and measure the peak-to-valley dose ratio that is consistent with other high spatial resolution detectors under the same irradiation conditions. The role of radioluminescence in the optical fibre used to transport the scintillation photons has been shown to create a significant contribution to the total light detected (Archer et al 2019).
m. The detector is able to resolve the individual microbeams, and measure the peak-to-valley dose ratio that is consistent with other high spatial resolution detectors under the same irradiation conditions. The role of radioluminescence in the optical fibre used to transport the scintillation photons has been shown to create a significant contribution to the total light detected (Archer et al 2019).
3.9. Diamond detectors
Recently a solid state diamond detector, potentially suitable for use as a dosimeter in MRT, have become commercially available (Livingstone et al 2015, Marinelli et al 2016, Butler et al 2018). The PTW model 60019 'microDiamondTM' (PTW-Freiburg GmbH, Freiburg, Germany) has a cylindrical active volume of 1.1 mm radius and 1  m length. The microDiamondTM is a synthetic single crystal diamond detector incorporating Schottky contacts, and is designed to be operated in passive mode with an electrometer (Almaviva et al 2010).
m length. The microDiamondTM is a synthetic single crystal diamond detector incorporating Schottky contacts, and is designed to be operated in passive mode with an electrometer (Almaviva et al 2010).
Operation within the extreme radiation environment (very steep dose gradients, high dose rate, kilovoltage energy spectrum) typically used in MRT is well outside the conditions of use recommended by the manufacturer. However, excellent results have been demonstrated, particularly in the penumbra regions of the microbeams (Livingstone et al 2015). Very careful and precise alignment of the microdiamond is required to achieve such results. The device should be operated in edge-on mode to utilize the 1  m thick active layer so as to fully exploit the best spatial resolution possible with this device.
m thick active layer so as to fully exploit the best spatial resolution possible with this device.
4. Dose calculation and treatment planning
4.1. Physics of dose absorption in microbeam radiation therapy
Dose calculation and its validation by experimental data can be performed with high accuracy in conventional RT. Relative dose uncertainties in conventional RT are usually below  (Brahme 1984, Mijnheer et al 1987) and dose validation is part of international standards (ICRU Report 24 1976). In MRT, compliance with such high standards is challenging. The small sizes of the radiation fields, large differences between peak and valley doses and the low photon energies place extraordinary high demands on dose calculation and dosimetry. In this section we present various approaches and physical prerequisites for dose calculations in MRT.
(Brahme 1984, Mijnheer et al 1987) and dose validation is part of international standards (ICRU Report 24 1976). In MRT, compliance with such high standards is challenging. The small sizes of the radiation fields, large differences between peak and valley doses and the low photon energies place extraordinary high demands on dose calculation and dosimetry. In this section we present various approaches and physical prerequisites for dose calculations in MRT.
MRT typically uses polychromatic x-ray beams with photon energies at around 100 keV. At these energies photons interact via photoelectric absorption, atomic Compton and Rayleigh scattering. Compton scattering is the most frequent photon interaction. Whereas MeV photons transfer the bulk part of their energy into kinetic energy of secondary electrons, the average energy transfer at low photon energy is rather low between 5 and  . Therefore multiple photon scattering substantially contributes to the scatter dose. Photoelectric absorption transfers almost all of the photon energy into kinetic energy of a secondary electron. The absorption coefficient of the photoelectric interaction strongly increases with decreasing photon energy and is particularly important for materials with atoms of higher atomic numbers.
. Therefore multiple photon scattering substantially contributes to the scatter dose. Photoelectric absorption transfers almost all of the photon energy into kinetic energy of a secondary electron. The absorption coefficient of the photoelectric interaction strongly increases with decreasing photon energy and is particularly important for materials with atoms of higher atomic numbers.
Secondary electrons generated in photon interactions of primary, i.e. unscattered photons deposit their kinetic energy predominantly within the microbeam peak regions, due to short electron ranges. The dose in the valley is caused by electrons of primary photons scattering out of the peak region and electrons generated in interactions of scattered photons. If the spacing between microbeams is sufficiently high, i.e. higher than the typical electron range, the PVDR is closely proportional to the ratio of peak width to peak pitch (Hugtenburg et al 2010, Donzelli et al 2018)
The absorption coefficient of 100 keV photons is around 5 times higher than for 5 MeV photons. Hence depth dose curves are considerably steeper and the range of secondary electrons is much shorter than 1 mm. The build-up effect, which dominates the first centimeters of conventional MeV-photon RT depth dose curves affects 1 mm or less of the depth dose curves for 100 keV photon beams.
The choice of appropriate photon energies in MRT needs to balance between short electron ranges guaranteeing sharp beam penumbras and shallower depth dose curves allowing to irradiate deeper targets without an excess of surface dose. A shift of the photon spectrum to higher photon energies in a broad wiggler spectrum is usually achieved by adding additional filters. However, these filters also reduce the dose rate. The electron range does not gradually increase with photon energy but depends on the ratio between Compton and photoelectric effect. Since Compton electrons receive only part of the initial photon energy, their range is much shorter than that of photo electrons. Therefore beam penumbras decrease with increasing energies below and increase again above 100 keV photon energy. At 200 keV the Compton electron range reaches around 25  m. A further increase of photo energy leads to a reduction of peak dose and PVDR of 50
m. A further increase of photo energy leads to a reduction of peak dose and PVDR of 50  m wide beams. While photon energy is less relevant in small animal studies, future clinical applications may require slightly higher photon energies between 150 and 200 keV.
m wide beams. While photon energy is less relevant in small animal studies, future clinical applications may require slightly higher photon energies between 150 and 200 keV.
4.2. Monte Carlo dose calculation
In the past, the majority of MRT dose calculations were performed with Monte Carlo techniques. Early dose estimates involved homogeneous and simplified phantoms and in parts mono-energetic photon beams (Stepanek et al 2000). A wide range of different Monte Carlo codes has been used for dose calculations. First dose calculations were performed by Slatkin et al (1992) using an early EGS4 (INHOM) (Nelson et al 1985) version that includes transport of photons, electrons and delta-rays. However, only total ionization cross-sections were used in the electron transport ignoring the distribution of scattering angles and energies. Simulations in later EGS4 versions (Orion et al 2000, De Felici et al 2005, 2007) showed substantial deviations to these early calculations. Later Monte Carlo calculations used the PENELOPE framework (Martínez-Rovira et al 2012b, Prezado et al 2012a), a GEANT3 PSI-version (Stepanek et al 2000), GEANT4 (Spiga et al 2007a, Bartzsch et al 2014, Cornelius et al 2014), EGS5 (Hugtenburg et al 2010) and MCNPX (Hanson et al 2013). De Felici et al (2008) performed a comparison of different Monte Carlo codes and did not find differences in the dose distributions calculated with GEANT4, EGS4, EGS-NRC, PENELOPE and MCNPX. Due to flexibility and accuracy, Monte Carlo techniques have become the standard in MRT dose calculation.
Important parameters for precise Monte Carlo simulations are energy cut-off values for electron tracking and the choice of scattering cross section libraries. Particularly at low photon energies the shape of microbeam penumbras and the valley dose depend on the choice of physical models (Spiga et al 2007b). When working with synchrotron radiation, the chosen physics libraries should account for polarization effects. At the spatial scales of several micrometres condensed history simulations of the electron scattering are sufficient. Only at smaller volumes track structure simulations with tools such as GEANT4-DNA become necessary (Lazarakis et al 2018).
Small voxel sizes are a challenge for Monte Carlo simulations. The probability that a voxel is hit by a particle is proportional to its volume. In order to keep the statistical uncertainty constant the number of particle histories needs to be scaled inversely proportional to the voxel volume. In contrast to conventional RT, where voxel sizes of approximately 1 mm are sufficient, a multiport MRT treatment may need around 5  m resolution in all spatial dimensions. This would require
m resolution in all spatial dimensions. This would require  times more particle histories and also memory than for Monte Carlo dose calculations in conventional radiotherapy. Therefore straight forward Monte Carlo simulations will not be feasible for MRT in the near future and strategies to overcome these challenges have to be provided. The majority of MRT dose calculations were performed for unilateral exposures and hence the voxel size can be reduced in only one spatial dimension (Siegbahn et al 2006, Martínez-Rovira et al 2012b). Other approaches use larger binning sizes and score peak and valley doses separately (Hugtenburg et al 2010, Debus et al 2017, Donzelli et al 2018), because anatomical information in a planning CT are provided on a coarse millimeter sized grid.
times more particle histories and also memory than for Monte Carlo dose calculations in conventional radiotherapy. Therefore straight forward Monte Carlo simulations will not be feasible for MRT in the near future and strategies to overcome these challenges have to be provided. The majority of MRT dose calculations were performed for unilateral exposures and hence the voxel size can be reduced in only one spatial dimension (Siegbahn et al 2006, Martínez-Rovira et al 2012b). Other approaches use larger binning sizes and score peak and valley doses separately (Hugtenburg et al 2010, Debus et al 2017, Donzelli et al 2018), because anatomical information in a planning CT are provided on a coarse millimeter sized grid.
4.3. Simulation of radiation sources
An accurate description of synchrotron beam properties such as phase space and spectrum are a prerequisite for accurate dose calculation. Several early Monte Carlo studies assumed ideally parallel microbeams with equal beam intensity. Nettelbeck et al (2009) investigated the influence of beam divergence and the collimator on the simulated microbeams. They realized an increase in penumbral dose of up to 26% due to beam divergence, although differences in the peak and valley centre disappeared. Martínez-Rovira et al (2012b) did a complete simulation of the medical beam line of the ESRF from the wiggler to the multislit collimator and used the phase space for subsequent dose calculations.
Bartzsch et al (2014) characterized the phase space and showed that a simplified model of the phase space leads to microbeam dose estimates that do not differ measurably from a full phase space simulation. This model assumes parallel beams within the phantom or patient, leakage radiation in the valley regions behind the absorber material with a different spectrum and accounts for a change of flux due to partial shadowing and the lateral profile of the synchrotron beam intensity.
A special feature of synchrotron radiation is its almost total linear polarization which impacts on the atomic Compton and Rayleigh scattering of photons in matter. Since Rayleigh scattering leads only to small angle deviations of photon trajectories and is not creating secondary electrons, polarization will mainly influence dose distribution via Compton interaction. The differential scattering cross section of the Compton effect is given by the Klein–Nishina Formula
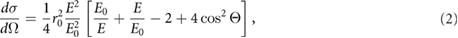
where E and E0 are the photon energies of incoming and scattered photon, r0 is the classical electron radius and  the angle between the polarization directions of incoming and scattered photon. Photons are preferentially scattered perpendicular to the polarization direction. One of the first investigating the effect of polarization were Orion et al (2000) using EGS4. Also De Felici et al (2005) used EGS4 to investigate how polarization effects the PVDR. They used 25
the angle between the polarization directions of incoming and scattered photon. Photons are preferentially scattered perpendicular to the polarization direction. One of the first investigating the effect of polarization were Orion et al (2000) using EGS4. Also De Felici et al (2005) used EGS4 to investigate how polarization effects the PVDR. They used 25  m wide beams, 200
m wide beams, 200  m centre-to-centre spacing and 30 mm
m centre-to-centre spacing and 30 mm  30 mm fields in a homogeneous water cylinder. Within the accuracy of the Monte Carlo simulations they did not see any polarization effects within the radiation field. Only in the photon scattering outside the microbeam field differences of up to 10% were observable. Hugtenburg et al (2010), on the other hand, came to the conclusion that polarisation effects are indeed essential for any future MRT treatment planning.
30 mm fields in a homogeneous water cylinder. Within the accuracy of the Monte Carlo simulations they did not see any polarization effects within the radiation field. Only in the photon scattering outside the microbeam field differences of up to 10% were observable. Hugtenburg et al (2010), on the other hand, came to the conclusion that polarisation effects are indeed essential for any future MRT treatment planning.
Studying the dose distribution around a pencil beam reveals how polarisation effects the dose distribution in microbeam fields (Spiga et al 2007b, Bartzsch et al 2014). Polarisation has a substantial impact on the direction of scattered Compton electrons and Compton photons and leads to a dose anisotropy. Photo electrons remain unaffected by photon polarisation and therefore dose absorption within the range of photo electrons is isotropic. As a consequence peak doses are almost unaffected by polarisation. Although the valley dose is dominated by Compton scattered photons, directional preferences level out within the microbeam field and polarisation corrections of the valley dose are between 1% and 3% in the field centre and field edge, respectively (Bartzsch et al 2014). Only in the scatter dose region outside the microbeam field substantial differences between polarized and unpolarized photons are observable (De Felici et al 2005, Hugtenburg et al 2010, Bartzsch et al 2014).
Despite considerable work and effort for precise dose calculations and dosimetry, substantial deviations between calculation and measurement have repeatedly been reported. Usually calculations are overestimating the PVDR and valley doses in measurements are 10 to 20% higher than predicted in simulations. Various effects may cause these deviations. Frequently discussed are influences of the multislit collimator. Although scattering from the collimator into the valley seems to be negligible (Nettelbeck et al 2009, Bartzsch et al 2014) simulations may induce unacceptable simplifications, such as perfectly plane surfaces. In fact a chemical analysis of the collimator surface of the biomedical beamline at the ESRF revealed substantial amounts of surface depositions, in particular copper. The fabrication process of the multislit collimator itself is challenging (Bräuer-Krisch et al 2009) and may infer variations in the peak width and peak distances which are not modelled in Monte Carlo simulations.
Furthermore Monte Carlo simulations neglect usually the wave nature of the particles they track, such as refraction, diffraction and total external reflection. Even hard x-rays have a refractive index which is slightly different to 1. At 100 keV this difference is in the order of  (Kuznetsov 2014) leading to a critical angle for total external reflection of around 0.8 mrad. Due to the low divergence of the synchrotron beam, this angle is large enough to cause total external reflection at the collimator walls even for the outermost beams of a 30 mm
(Kuznetsov 2014) leading to a critical angle for total external reflection of around 0.8 mrad. Due to the low divergence of the synchrotron beam, this angle is large enough to cause total external reflection at the collimator walls even for the outermost beams of a 30 mm  30 mm field. Future investigations are required to reveal the cause of remaining discrepancies between simulation and measurement.
30 mm field. Future investigations are required to reveal the cause of remaining discrepancies between simulation and measurement.
4.4. Alternative dose calculation methods
4.4.1. Semi-adjoint Monte Carlo simulations
As mentioned in section 4.2, the main problem when applying Monte Carlo tools to MRT are the required small voxel sizes. Monte Carlo problems with small detector volumes or when studying variable sources are often treated in their adjoint form Irving (1971) and Iván Lux (2000). Mathematically, Monte Carlo simulations can be seen as an integration of the Boltzmann transport equation (Iván Lux 2000). In the adjoint version of this integro-differential equation, detector and source term exchange their position. Hence, in the adjoint Monte Carlo simulation all 6 phase space dimensions of detector and source are swapped and interactions are tracked 'backwards in time'.
For MRT, the detector volume is small in the two dimensions perpendicular to the beam propagation. The MSC, as photon source has a large phase-space extension perpendicular to the propagation direction, while the source is small in all other phase space dimensions. Therefore the adjoint Monte Carlo problem will not lead to an advantage. However, it is possible to formulate a semi-adjoint version of the Monte Carlo problem exchanging only the two spatial dimensions perpendicular to the beam direction (see figure 7). As the momentum dimensions of detector and source remain unchanged in the phase space, particles can be tracked 'forward in time'. However, this partially adjoint version of the Boltzmann transport equation can only be derived if the phase space variables clearly separate, restricting this method to problems which are homogeneous perpendicular to the propagation direction and demand material homogeneity.
Figure 7. Forward (A) and semi-adjoint (B) Monte Carlo transport. An exchanging of source and detector geometry in the y − z-plane will lead to the same expected energy absorption in the detector, if the number of particles per source area is identical. Prerequisite is the homogeneity of the phantom in the y − z-plane.
Download figure:
Standard image High-resolution imageTheoretical derivations of this problem may be found in the respective literature dealing with Monte Carlo techniques and the Boltzmann transport equation. Here we only provide a brief heuristic explanation. Consider a photon emitted from the source element dS in the forward Monte Carlo problem in figure 7(A). The probability that a certain energy fraction dE of this photon is absorbed in the detector volume element  is equal to the probability that the same energy fraction dE of a photon leaving the semi-adjoint source element
is equal to the probability that the same energy fraction dE of a photon leaving the semi-adjoint source element  in figure 7(B) is absorbed in the semi-adjoint detector element
in figure 7(B) is absorbed in the semi-adjoint detector element  . More complicated source and detector geometries can be considered as compositions. If the number of particles emitted per source volume is equal for all source elements, the expectation of energy absorption in the forward detector and semi-adjoint detector will indeed be equal.
. More complicated source and detector geometries can be considered as compositions. If the number of particles emitted per source volume is equal for all source elements, the expectation of energy absorption in the forward detector and semi-adjoint detector will indeed be equal.
This method was employed by various authors (Hugtenburg et al 2010, Schültke et al 2013, Bartzsch et al 2014, 2015). It is particularly useful to determine so called relative output or scatter factors (ROF). With a single simulation it is possible to calculate ROFs for various field shapes. However, the restriction of this method to slab geometries limits its use in MRT treatment planning.
4.4.2. Kernel based dose calculation approaches and hybrid dose calculation
Despite a wide spread use of kernel based dose calculation algorithms in conventional RT for MeV photons, such algorithms are rarely used in the low energy x-ray domain below 1 MeV. In order to deal with tissue inhomogeneities, existing kernel based algorithms employ O'Connor's electron density scaling method (O'connor 1957, Mackie et al 1985, Bortfeld et al 1993), which is not applicable at low photon energies. Nevertheless, kernel based dose calculation methods for lower photon energies (Alaei et al 2000, 2010, Carlsson and Ahnesjö 2000) and also for MRT (Bartzsch and Oelfke 2013, Debus et al 2017) have been developed. Such kernel based dose calculation algorithms are capable of calculating microbeam dose distributions within five minutes and further acceleration seems technically feasible (Debus et al 2017). However, comparisons with Monte Carlo simulations show larger deviations, particularly in the valley regions.
Accurate and fast dose calculation can be achieved by combining kernel based and Monte Carlo based techniques in a hybrid approach. Problematic for kernel based dose calculation is the scattering of photons in inhomogeneous material. On the other hand, the tracking of secondary electrons on a micrometre scale is time consuming for Monte Carlo calculations. Photon scattering on a millimeter grid can be computed very efficiently, even within a few seconds as demonstrated in the past (Badal and Badano 2009, Jia et al 2010, 2012). Kernel algorithms can calculate the electron scattering with high accuracy assuming homogeneous material. Information on tissue inhomogeneities are given on a coarse millimeter sized grid.
In a hybrid dose calculation approach, Monte Carlo methods are used to calculate primary and scatter photon dose on a millimeter grid without considering electron scattering. In a subsequent electron convolution algorithm the microbeam pattern is calculated. Donzelli et al (2018) were able to show that such algorithms are around 600 times faster than pure Monte Carlo simulations and that there are no relevant differences in the calculated dose distributions when compared to pure Monte Carlo. Polarization effects and source phase space can be easily integrated into such hybrid methods.
4.5. Treatment planning
MRT treatment planning has to overcome several challenges compared to conventional RT. The dose needs to be calculated on very small spatial scales. As described above this requires specialized methods in dose calculation, but also storage and visualization of the calculated dose distribution deserves extra considerations. Quality assurance criteria such as the  index consider absolute dose changes or dose changes relative to the maximum dose (Low et al 1998). Since the Valley doses are only 5% of the peak dose, a valley dose accuracy of only 10% would require a very strict dose accuracy in the
index consider absolute dose changes or dose changes relative to the maximum dose (Low et al 1998). Since the Valley doses are only 5% of the peak dose, a valley dose accuracy of only 10% would require a very strict dose accuracy in the  -index of only 0.5%. Another challenge is the low photon energy and the related sensitivity to material composition. This requires special care when converting CT Hounsfield units into material composition for dose calculation.
-index of only 0.5%. Another challenge is the low photon energy and the related sensitivity to material composition. This requires special care when converting CT Hounsfield units into material composition for dose calculation.
Most preclinical studies in MRT used a few summary measures to characterize the dose distribution such as peak dose, valley dose or PVDR. Such measures can be calculated and visualized on a conventional millimeter sized grid. The definition of peak and valley dose requires some, often neglected attention, though. All dose measurements and calculations provide dose values on a finite grid and therefore some form of spatial averaging. However, because the doses vary on very small length scales, peak and valley doses should always be reported together with the applied averaging or voxel sizes for comparison. It would be desirable to establish standards on how peak and valley doses are presented.
The relevant radio-biological dose measure in MRT is a matter of ongoing research. Early studies in MRT usually used peak dose to compare biological results (Slatkin et al 1995, Laissue et al 2001, Serduc et al 2006, 2008), since peak dose is the easiest accessible quantity. More recent results show, however, that rather the valley dose is deciding on biological effects (Serduc et al 2009, Bouchet et al 2016), tumour control and tissue damage. A typical configuration of a microbeam exposure is 50  m wide beams with a spacing of 400
m wide beams with a spacing of 400  m. 'Perfect microbeams' with a sufficiently high peak dose and no valley dose, would kill 1/8th of the cells in the microbeam peaks and leave 7/8th of the cells in the valleys unaffected. A reduction of cell survival by 1/8th would correspond to only a few cGy homogeneous dose. Hence, the valley dose will mainly determine clonogenic cell survival if intercellular communication can be ignored.
m. 'Perfect microbeams' with a sufficiently high peak dose and no valley dose, would kill 1/8th of the cells in the microbeam peaks and leave 7/8th of the cells in the valleys unaffected. A reduction of cell survival by 1/8th would correspond to only a few cGy homogeneous dose. Hence, the valley dose will mainly determine clonogenic cell survival if intercellular communication can be ignored.
Under the assumption that the clonogenic cell survival is determining tissue damage or tumour control and that only dose decides upon the fate of a cell, i.e. no bystander mechanisms, the equivalent uniform dose (EUD) (Niemierko 1997) would be the optimal parameter for MRT treatment planning as suggested by Meyer et al (2017). The advantage of this measure is its independence of beam geometries. For any microbeam dose pattern, even in cross firing regions the EUD can be calculated upon voxel based dose volume histograms as suggested by Donzelli et al (2018) (see figure 8).
Figure 8. Voxel based dose volume histogram (right) for a cross firing geometry of microbeams (left).
Download figure:
Standard image High-resolution imageDose calculation algorithms have been coupled to TPS as in conventional RT. Such systems allow to define target volumes in CT-images of future patients, the adjustment of beam parameters such as field size, filtering, beam direction, microbeam width and spacing and the visualisation of calculated treatment doses and parameters. The first MRT TPS was based on a PENELOPE Monte Carlo dose calculation engine (Martínez-Rovira et al 2012a). Debus et al (2017) presented the TPS VIRTUOS (Bendl et al 1994) with a kernel based dose calculation engine. Recently a hybrid dose calculation engine has been coupled to the popular TPS Eclipse (Varian) (Poole et al 2017) (see figure 9).
Figure 9. Treatment planning in preparation for pet clinical trials at the Australian Synchrotron with a combination Eclipse and the hybrid dose calculation.
Download figure:
Standard image High-resolution image5. Multimodal MRT
While microbeam radiotherapy produces a unique way of selectively targeting cancerous tissues, further normal tissue sparing or tumour radio-sensitivity enhancement could be achieved with a multimodal approach combining MRT with nanoparticles (NPs) and/or chemotherapies.
The idea that the therapeutic index of MRT could be further improved by combining MRT irradiation with dose enhancers was first proposed by Dilmanian et al (2007). Synchrotron MRT beams in the 100 keV range are naturally prone to large absorption cross sections in elements with high atomic numbers, increasing secondary electron production associated mainly with the photoelectric effect. Contrast agents such as iodine, gadolinium or gold were proposed as possible candidates. Monte-Carlo simulated dose enhancement factors for MRT were obtained for different iodine, gadolinium and gold concentrations. A clear superiority was shown for gadolinium and gold with respect to iodine for dose enhancement in a human head phantom geometry (Prezado et al 2009). Other elements like thallium, lutetium or hafnium proved to also give satisfactory enhancements depending on the geometry of the irradiation (Martínez-Rovira and Prezado 2011).
If in silico analysis emphasized the interdependent roles of irradiation geometry, synchrotron beam energy and the choice of contrast agent material, the reality of in vitro and in vivo experiments add extra layers of constraints in the optimization problem. Tumour selectivity and specificity, injection modes and timing, contrast agents' size, micro- and macroscopic distribution, toxicity and retention, all become parameters of critical importance together with the choice of cell lines and animal models. Experimental verification of predicted dose enhancements is however crucial to demonstrate efficiency and better understand the underlying physical, chemical and biological mechanisms of interactions between the synchrotron microbeams and the dose enhancers.
Among other metallic high-Z NPs, gold NPs seem to be privileged candidates for multimodal MRT due to their relatively low intrinsic toxicity, high biocomptability and their capacity to diffuse and concentrate in tumours. Gokeri et al (2010) simulated that the hypothetical presence of 7 mg of gold per gram of tumour in a realistic head phantom irradiated with synchrotron microbeams would lead to substantial target dose increase allowing reduced skin, skull bone and maximum brain doses to produce the desired target dose. This dose increase was experimentally evidenced using normoxic polyacrylamide gels and bovine aortic endothelial cells exposed to 1mMol l−1 gold NPs (Rahman et al 2010). Interestingly, the gold NPs seem to influence the recovery rate of eradicated area in vitro, with a distinct behavior in favor of normal cells compared to cancerous ones which emphasizes once more a differential effect of MRT (Rahman et al 2011). In an in vivo experiment, Miladi et al (2014) showed that gold NPs coated by gadolinium chelates improved survival of synchrotron MRT treated rats bearing intracerebral 9L gliosarcoma (9LGS), an extremely radioresistant tumour.
Among the strong candidates, gadolinium based NPs are currently gaining popularity worldwide with the advances of image guided radiotherapy, in particular MRI guided radiation therapy delivery systems. Beyond their paramagnetic properties, the presence of the high-Z gadolinium atom makes them suitable as radiation dose enhancer. This was evidenced in vivo by Le Duc et al (2011) where rats bearing intracerebral 9LGS had their survival increased by a factor of five when gadolinium NPs were intravenously administered 20 minutes before their MRT treatment. Le Duc et al (2014) further demonstrated that more conventional gadolinium chelates did not appear to be of any benefit compared to AGuIX®, a gadolinium-based NP, that has now reached clinical trials with conventional radiation beams. Further optimization of the time sequence showed the radiosensitizing effect of the gadolinium NPs is increased when MRT is delivered 24 h after injection, when the tumoral and cellular distributions of the NPs maximize lethal effects (Dufort et al 2016).
The importance of NPs' cellular distribution, especially in the unique spatially fractionated dose distributions of synchrotron microbeams, was also emphasized by Engels et al (2016) for the specific example of Tantalum pentoxide NPs. These non-toxic, nano-structured ceramic compounds were recently introduced as possible radiosensitizer and observed to form shells around cell nuclei instead of distributing homogeneously in the medium, producing remarkably different physical dose enhancements. This study showed that NP dose enhancement for synchrotron MRT is highly dependent on the NP congregation properties, location of congregations with respect to the beam peak, and photon energy.
In order to further improve the radiotherapeutic efficacy of MRT, other methods were developed by combining the effects of existing or novel anti-cancer drugs. If existing chemotherapy drugs like cisplatinum or temozolomide did not significantly improve the lifespan of the MRT treated rats with intracerebral 9L gliosarcoma, synergetic effects were observed in vivo using tubulin polymerization inhibitor JAI-51 (Bouchet et al 2012). The enhanced radio-sensitization of this antimetabolic drug was strongly correlated to G2/M phase cycle arrest. A major contributor to MRT efficacy is the immune response modulation (Bouchet et al 2013b). Smilowitz et al (2006) demonstrated that gene-mediated immunotherapy provides an important synergetic effect when combined with MRT, with almost 50% of the treated rats being long term survivors (>1 year). The preferential action of MRT on vascular network also lead to some other intrinsically linked radiation sensitization, immune function or chemotherapy efficacy improvement mechanisms, e.g. tumour oxygenation, which could be further exploited with anti-angiogenic agents (Griffin et al 2012).
In a more holistic approach, simultaneous use of chemotherapy drugs and NPs also opens the door to more complex theranostics aimed at image-guided and targeted selective lethal damages enhancement. Recent advances in the field of cancer therapy are focused on the design of novel drug delivery systems that feature therapeutic, diagnostic and imaging capabilities simultaneously. MRT will undoubtedly benefit from these advances.
6. Novel and future radiation sources
One of the main obstacles to the clinical translation of MRT is a lack of compact microbeam sources. Currently only a few large synchrotrons worldwide seem to be capable of generating the required beam properties. It is commonly accepted that the benefit of MRT over conventional RT critically depends on high PVDRs, low beam penumbras and sufficiently shallow dose fall-off with depth in order to treat deep seated tumours. Only radiation qualities that show little lateral scattering and generate short-ranged secondary particles are able to meet these criteria. Photon beams with kinetic energies between 100 keV and 300 keV seem to offer an acceptable compromise between low lateral scattering and low peak entrance doses when targeting deep seated tumours.
Apart from an appropriate radiation quality the source needs to provide low divergence and a source dimension which is smaller than the size of the generated beams, to ensure constant beam profiles with distance from the collimator and to keep beam penumbras small. Particularly challenging is the conservation of the micrometre sized dose profiles under the conditions of cardiovascular and respiratory motion in the traversed tissue. Radiation doses have to be applied within fractions of a second. Only a few large third generation synchrotrons are currently able to provide such high dose rates. However, radiobiological evidence for the requirement of dose rates in the 10 kGy range is still missing and in vivo experiments at lower dose rates have already successfully been carried out.
Third generation synchrotrons are large research facilities, with limited capacity for clinical studies in MRT and they are too expensive to be dedicated to cancer therapy alone. If MRT is to be established as a widespread radiotherapy treatment option, alternative compact microbeam sources need to be developed. Synchrotrons may demonstrate the principle feasibility of MRT, but are unlikely to provide widespread clinical applications.
6.1. Inverse Compton scattering sources
Promising and frequently discussed alternative radiation sources in MRT are inverse Compton scattering sources such as the Munich compact light source (Eggl et al 2016, Wright 2015). The principle of inverse Compton scattering is similar to synchrotrons. Instead of periodically deflecting electrons in static magnetic fields of undulators or wigglers, electrons interact with the electric field of a strong laser. Because the wavelength of emitted x-ray photons scales with the period of the wiggler field, lower electron energies are required to generate hard x-rays. The energy Ex of the emitted x-rays is approximately given by Loewen (2004)

with a narrow spectrum.
Microbeams have been produced at the Munich Compact Light Source (MuCLS), the first commercially sold inverse Compton scattering source (Lyncean Technologies Inc., USA). The first in vitro studies were published (Burger et al 2017a) and currently in vivo studies in mice are being carried out (Burger et al 2017b, Dombrowsky et al 2019). MuCLS operates with a 4.6 m circumference storage ring with electrons of up to 45 MeV kinetic energy and produces photon energies between 15 and 35 keV. The source diameter is around 42  m. First preclinical in vitro experiments used 25 keV photons with a dose rate of 1 Gy min−1 at 1.7 m distance from the source. Due to the low photon energies a 200
m. First preclinical in vitro experiments used 25 keV photons with a dose rate of 1 Gy min−1 at 1.7 m distance from the source. Due to the low photon energies a 200  m thick tungsten foil with 50
m thick tungsten foil with 50  m wide slits was sufficient to collimate microbeams with a pitch of 350
m wide slits was sufficient to collimate microbeams with a pitch of 350  m (Burger et al 2017a).
m (Burger et al 2017a).
The future usability of inverse compton scattering sources for clinical applications in MRT depends on the possibility to upscale flux and photon energy of current machines. There are currently two designs investigated, linear accelerator based systems, which produce a slightly higher brilliance and storage ring based systems, such as MuCLS. Although linear accelerator based system offer a higher brilliance, storage ring based systems seem to be better suited for radiation therapy.
Despite substantial progress in the last years, Jacquet and Suortti (2015) estimate that even for ThomX, one of the most advanced systems being currently developed, the achieved dose rate will be in the order of a few Gy/min at an energy of up to 90 keV for clinically relevant field sizes. Table 2 shows currently developed inverse Compton scattering sources and their parameters.
Table 2. Inverse Compton scattering source projects with sources that provide more than 1012 ph s−1. SR stands for storage ring, Linac for linear accelerator. The dose rate has been approximated assuming a field size of 20 mm2  20 mm2. Table adapted from Jacquet and Suortti (2015), reproduced with permission from Elsevier.
20 mm2. Table adapted from Jacquet and Suortti (2015), reproduced with permission from Elsevier.
| Project | Place | Type | EX (keV) | Flux (ph s−1) | Source size (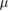 m) m) |
Dose rate (Gy s−1) |
|---|---|---|---|---|---|---|
| TTX | Beijing, China | SR | 20–80 | 1012 | 50 | 0.08 |
| NESTOR | Kharkov, Ukraine | SR | 30–500 | 1013 | 70 | 6.6 |
| ThomX | Orsay, France | SR | 20–90 | 1013 | 70 | 0.9 |
| KEK QB | Tsukuba, Japan | Linac | 35 | 1013 | 10 | 1.5 |
| KEK ERL | Tsukuba, Japan | Linac | 67 | 1013 | 30 | 0.8 |
| MIT | Cambridge, USA | Linac | 3–30 | 1014 | 2 | 19 |
6.2. Compact x-ray tube based microbeam sources
Conventional x-ray tubes are an abundantly available and inexpensive source of x-rays. In x-ray tubes electrons are accelerated to kinetic energies of up to several hundred keV and hit a target made of materials with high atomic numbers (usually tungsten). Interactions between electrons and target atoms generate bremsstrahlung and characteristic x-ray photons, which are emitted almost isotropically into a large solid angle. The efficiency of the conversion of electron beam energy into x-ray energy is very low in the order of around 1%. Most of the kinetic electron energy is converted into heat. To keep the surface temperature of the target below the melting point, the intensity of the electron beam in the focal spot is limited leading to a trade-off between focal spot (source) size and flux. Typical spot sizes are in the order of a few millimetre. As x-ray tubes deliver a strongly divergent x-ray beam the dose rate is comparably low at reasonable distances from the focal spot. Hence, when using x-ray tubes for MRT, beam divergence close to the source, small relative output factors (ROF) and partial shadowing behind the collimator openings impair the microbeam field.
Several investigators developed compact microbeam systems for preclinical research (Babcock et al 2011, Bazyar et al 2017). However, most of these preclinical systems have larger aperture widths and should therefore rather be classified as minibeam systems. Fabrication of appropriate collimators is one of the most difficult steps in the development of x-ray tube based microbeam irradiators.
Recently, small animal radiotherapy platforms were developed to provide clinical standards in preclinical radiotherapy research, e.g. by establishing image guidance and treatment planning tools (Wong et al 2008). Such preclinical radiotherapy plattforms have recently also been used for the production of microbeams (or minibeams). Prezado et al (2017a) described a SARRP (Xstrahl Ltd, UK) based system with a 30 mm thick brass collimator that produced 400 to 500  m wide beams with a centre to centre distance of around 1200
m wide beams with a centre to centre distance of around 1200  m accounting for beam divergence. As the dose rate at the isocentre was too low they moved to 20 cm source distance and achieved 3.5Gy min−1 with a PVDR of around 12. With such a system they were able to achieve 58 Gy peak dose in a preclinical study with rats. In order to further increase the dose rate, they moved the sample off the isocentre closer to the source. Thus, unfortunately many of the features in the small animal radiotherapy platform are lost.
m accounting for beam divergence. As the dose rate at the isocentre was too low they moved to 20 cm source distance and achieved 3.5Gy min−1 with a PVDR of around 12. With such a system they were able to achieve 58 Gy peak dose in a preclinical study with rats. In order to further increase the dose rate, they moved the sample off the isocentre closer to the source. Thus, unfortunately many of the features in the small animal radiotherapy platform are lost.
Esplen et al (2018) investigate possibilities to produce finer microbeams with 100 to 200  m width at the SARRP isocentre and also tested a very simple collimator made of steel septa and double sided tape that produces 135
m width at the SARRP isocentre and also tested a very simple collimator made of steel septa and double sided tape that produces 135  m wide beams. They estimate the achievable peak dose to be roughly 90 Gy, assuming anesthesia to be limited to 1 h maximum.
m wide beams. They estimate the achievable peak dose to be roughly 90 Gy, assuming anesthesia to be limited to 1 h maximum.
A set-up that produces the typical microbeam configurations achieved at synchrotrons was presented by Bartzsch et al (2016). The authors used a tungsten collimator with divergent slits and achieved a dose rate of 10–18 Gy min−1 in only 6 cm distance from the focal spot of a 160 kVp x-ray tube. The PVDR of the 50  m wide and 400
m wide and 400  m spaced beams was as high as 30, but depended strongly on the distance to the collimator. Due to the large divergence of the produced microbeams, the system was dedicated to in vitro research only.
m spaced beams was as high as 30, but depended strongly on the distance to the collimator. Due to the large divergence of the produced microbeams, the system was dedicated to in vitro research only.
6.3. Advanced x-ray tube technology
6.3.1. Carbon nanotube field emission technology
Schreiber and Chang (2012) investigated carbon nanotube field emission technology (CNT) as a mean to produce microbeams with x-ray tubes. In contrast to conventional thermionic electron emission requiring high cathode temperatures, CNT technology exploits high electric fields close to nanometer sized cathode structures to extract electrons at room temperature. The advantage of CNT cathodes over conventional thermionic cathodes are the achievable high electron current densities and small emittance (Shiffler et al 2004). Schreiber and Chang (2012) proposed a ring arrangement of anode segments around the patient as shown in figure 10. The electron beam is shaped to match the size of the projection of the collimator opening on the anode surface. A 10 cm thick single slit collimator with 100  m slit width produced a single microbeam and by vertical translation between source and target several parallel microbeams can be applied. In Monte Carlo simulations they calculated the dose for a ring assembly of 24 cathode segments and estimated that such an assembly could deliver up to 280 Gy s−1. Due to the ring geometry the entrance dose can be substantially reduced and reaches only 10% of the target dose. Moreover, they showed that a reduction of photon energy from 225 kVp to only 100 kVp has only minimal impact on the dose in the isocentre.
m slit width produced a single microbeam and by vertical translation between source and target several parallel microbeams can be applied. In Monte Carlo simulations they calculated the dose for a ring assembly of 24 cathode segments and estimated that such an assembly could deliver up to 280 Gy s−1. Due to the ring geometry the entrance dose can be substantially reduced and reaches only 10% of the target dose. Moreover, they showed that a reduction of photon energy from 225 kVp to only 100 kVp has only minimal impact on the dose in the isocentre.
Figure 10. Ring geometry of carbon nanotube field emission technology x-ray tube developed by Schreiber and Chang (2012). The distribution of the source allows to create high peak doses in the isocentre while keeping the peak entrance dose low. Figure from Schreiber and Chang (2012) reproduced with permission of John Wiley & Sons.
Download figure:
Standard image High-resolution imageHadsell et al (2013) presented a first prototype of a system with a 0.14 mm wide and 162 mm long focal track. With an acceleration voltage of 160 kV, 70 mA anode current they achieved a dose rate of 2 Gy s−1 in pulses of 0.1 s. A collimator produces a 300  m wide beam. They apply these beams with 900
m wide beam. They apply these beams with 900  m centre-to-centre spacing in a mouse experiment by shifting the sample holder and achieved 13 Gy peak entrance dose with a PVDR of 17. In another study they also demonstrate the feasibility of the ring shaped set-up (Hadsell et al 2014), although at much lower photon energy and dose rate.
m centre-to-centre spacing in a mouse experiment by shifting the sample holder and achieved 13 Gy peak entrance dose with a PVDR of 17. In another study they also demonstrate the feasibility of the ring shaped set-up (Hadsell et al 2014), although at much lower photon energy and dose rate.
However, until now there is no clinical system available and the microbeam width is rather in the minibeam domain. Whether such broader beams provide the same medical benefit as thinner beams needs to be validated. Moreover, the application of larger field in human sized targets may lead to substantially reduced PVDRs.
6.3.2. Line focus x-ray tube
Another recently developed concept is termed line focus x-ray tube (Bartzsch and Oelfke 2017). This concept suggests similarly a strongly eccentric focal spot on a rapidly rotating target. Monte Carlo simulations showed that such a system could provide a dose rate of 180 Gy s−1 in 0.5 m distance from the focal spot. At very high target surface velocities and high acceleration voltages, electron scattering is the dominant energy transport in the target instead of heat conduction. Consequently, heat capacity alone decides upon the focal spot temperature and a change in the focal spot width does not influence its temperature. Hence the focal spot width is only limited by lateral scattering of the electrons.
Due to the small focal spot width the ROF becomes approximately 1. For a clinical system, Bartzsch et al (2016) suggested an acceleration voltage of 600 kV at a power of 1.5 MW. The maximum pulse length was estimated to be around 4 s delivering more than 700 Gy peak entrance dose. The higher mean photon energy of 150 keV will be advantageous for patient treatment due to a higher penentration depth. However, an experimental proof of concept remains outstanding.
6.4. Proton microbeams
Although research in MRT has primarily focused on photons, also particles have been investigated as a source for microbeams. Dilmanian and Meek (2010) proposed to use heavy ions for MRT but excluded protons from their patent, because they show strong lateral scattering. Nevertheless, particularly protons have recently gained attention, as proton beams are relatively easy to produce and shape. With depth these proton microbeams widen and the spatial dose modulation is lost in the Bragg peak region. In practice tissue sparing by dose modulation is achieved in the beam entrance region, whereas the tumour is treated with a conventional homogeneous dose as shown in figure 11. Hence, the technique is very similar to conventional proton therapy but with an additional normal tissue sparing effect in the beam entrance region.
Figure 11. Simulated dose distribution of 60 MeV proton microbeams with 100  m width and 1 mm spacing; figure from Kłodowska et al (2015) reproduced with permission of Elsevier. The phantom was either a homogeneous water cylinder (red) or a water cylinder with a bone slab (black). Dose distributions are normalised to their maximum. The background shows the mean depth dose curve.
m width and 1 mm spacing; figure from Kłodowska et al (2015) reproduced with permission of Elsevier. The phantom was either a homogeneous water cylinder (red) or a water cylinder with a bone slab (black). Dose distributions are normalised to their maximum. The background shows the mean depth dose curve.
Download figure:
Standard image High-resolution imageZlobinskaya et al (2013) were the first to introduce spatially fractionated radiation therapy with protons and proposed so called micro-channels: 10–50  m wide pencil beams irradiated in a two dimensional grid with 500
m wide pencil beams irradiated in a two dimensional grid with 500  m spacing. Preclinical studies have shown that proton micro-channels lead to a similar tissue sparing effect as photon microbeams (Girst et al 2015).
m spacing. Preclinical studies have shown that proton micro-channels lead to a similar tissue sparing effect as photon microbeams (Girst et al 2015).
Prezado and Fois (2013) investigated the possibility to use high energy protons of 1 GeV for the production of microbeams or minibeams. Since the range of 1 GeV protons is substantially larger than the size of a patient, the spatial modulation is preserved throughout the patient. However, currently only the Petersburg Nuclear Physics Institute, Russia, operates a synchrocyclotron capable of producing 1 GeV protons for medical applications. It is questionable whether laser accelerated particle sources will ever be compact and powerful enough to provide a reasonable alternative.
7. Conclusions
Access to suitable synchrotron light sources has limited MRT research to a small research community and has complicated the process of clinical translation until now. However, over time, a wealth of pre-clinical data has been acquired that impressively demonstrates the efficacy of microbeams in cancer treatment and also in neurological disorders such as epilepsy. The safe and responsible treatment of patients with microbeams requires solutions to several technological and medical physics challenges in MRT which are related to: the micrometre scale and gradient of the beams, the extremely high dose rates at synchrotrons, the low photon energy, and the development of compact alternative x-ray sources. This article reviewed these challenges and existing solutions. Despite these challenges, powerful dosimetry and dose calculation tools have been developed for MRT and are ready to use for first veterinary and human applications.
The need for treatment planning of micrometre sized radiation fields has led to the development of a range of dose calculation methods reviewed in chapter 4. Although Monte Carlo simulations are the standard for MRT dose calculation, the spatial dimensions required to score radiation doses render straight forward Monte Carlo solutions impractical for clinical applications. Appropriate scoring methods can reduce the number of required particle histories and curtail the amount of data. There exist also some alternative approaches to Monte Carlo techniques; particularly hybrid methods combining accurate Monte Carlo tools with fast convolution algorithms seem attractive.
A matter of debate is the clinically relevant radiation quantity that should be used for treatment planning, e.g. peak dose, valley dose or integrated dose. A promising approach may be the equivalent uniform dose (EUD) which relates inhomogeneous dose distributions to a homogeneous dose that would lead to equivalent cell survival. A final solution to this question requires further preclinical data and radiobiological models.
The high dose rate at synchrotrons poses a safety risk for patients, which needs to be handled reliably. Interlock systems and beam switches need to operate at high speed. At the European Synchrotron a patient safety system (PASS) was developed combining monitoring and shutter systems to handle these risks. To keep microbeam penumbras sharp, low photon energies of around 100 keV are used in MRT. The downside of such low photon energies are steep dose fall-offs with depth, known from early days of radiotherapy and treatments with orthovoltage radiation. A possible solution to this problem may be a concomitant treatment with dose enhancers such as gold or gadolinium nanoparticles, discussed in section 5. For treatment of patients the chosen photon energy spectrum needs to be a compromise between sharp beam penumbras and reasonable penetration depth.
Synchrotrons are likely to be the first place for clinical trials in MRT. Currently, the Australian and the European Synchrotron are investing in veterinary MRT trials as an intermediate step towards clinical applications in human patients. The purpose of veterinary trials—treating pet animals suffering from spontaneously growing tumours—is two-fold. Firstly, essential radiobiological data will be collected for both normal tissue toxicity and tumour control on a scale more closely resembling humans. Secondly, technological advancements, including treatment planning, image-guidance and patient positioning systems, will be tested, validated and refined for future clinical use.
In the long-term, clinical trials at large, synchrotron research facilities are logistically demanding. The widespread clinical use of MRT at synchrotrons is unlikely, although not impossible. Several promising alternative sources are under development and are already used for pre-clinical research. In the future, such sources could provide clinically suitable microbeams in a conventional hospital environment and convert MRT from an experimental method into a routinely deliverable treatment option. Furthest progressed are x-ray tube based concepts such as carbon nanotube cathode technology and line focus x-ray tubes, as they achieve dose rates of more than 100 Gy s−1 with appropriate photon energies.
To conclude, the promising therapeutic features of MRT are intrinsically tied to its distinct and demanding physical properties. The field of MRT has a clear clinical trajectory, however, the fulfilment of this pathway largely relies on advancements in medical physics. Although challenges undoubtedly remain, significant and encouraging progress has been made. With the continued development of suitable solutions for the challenging physics of x-ray microbeams, the first veterinary and human trials of MRT are within reach.
Acknowledgment
We dedicate this review article to the memory of Dr Elke Bräuer-Krisch (BE, PhD) who sadly passed away on 10 September 2018.
Elke was a central figure in Synchrotron MRT research and was known around the world for her outstanding contributions to the field, in particular MRT dosimetry.