Abstract
Purpose
To demonstrate the potential of monitoring H/D exchange by FT-Raman spectroscopy as a tool for the detection and quantification of low levels of amorphous lactose in formulations.
Methods
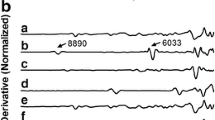
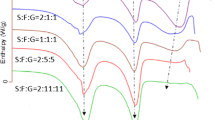
Samples containing different proportions of amorphous and crystalline lactose were prepared. H/D exchange was carried out by exposing the samples to a flow of D2O vapour. A calibration curve was constructed from the FT-Raman spectra of the deuterated samples by integrating the ν(OD) band and normalizing to an internal standard. This method was benchmarked against a conventional approach using Raman spectroscopy where the ratio of Raman bands associated with crystalline and amorphous lactose is used to estimate the amorphous content.
Results
The H/D exchange method revealed a linear response over the entire composite range with an excellent correlation coefficient (R 2 = 0.999). The sensitivity of this approach in detecting the amount of amorphous lactose present in a blend is significantly greater than that offered by conventional FT-Raman in the 0–10% level of amorphous material.
Conclusions
A non-destructive method that is capable of providing reproducible measurements of low levels of amorphous material in lactose has been demonstrated and this method has enhanced sensitivity relative to approaches using Raman spectroscopy without deuteration.








Similar content being viewed by others
References
B. C. Hancock, and G. Zografi. Characteristics and significance of the amorphous state. J. Pharm. Sci. 86:1–12 (1997). doi:10.1021/js9601896.
A. T. Florence, and E. G. Saloloe. Changes in crystallinity and solubility on comminution of digoxin and observations on spironalactone. J. Pharm. Pharmacol. 28:637–642 (1976).
Y. Matsunga, N. Bando, H. Yuasa, and Y. Kanaya. Effects of grinding and tabletting on physico-chemical stability of anticancer drug, TAT-59. Chem. Pharm. Bull. 44:1931–1934 (1996).
M. Otsuka, and N. Kaneniwa. Effect of grinding on the degree of crystallinity of cephalexin powder. Chem. Pharm. Bull. 31:4489–4495 (1983).
A. Saleki-Gerhardt, C. Ahlneck, and G. Zografi. Assessment of disorder in crystalline solids. Int. J. Pharm. 101:237–247 (1994). doi:10.1016/0378-5173(94)90219-4.
D. Q. M. Craig, P. G. Royall, V. L. Kett, and M. L. Hopton. The relevance of the amorphous state to pharmaceutical dosage forms: glassy drugs and freeze dried systems. Int. J. Pharm. 179:179–207 (1999). doi:10.1016/S0378-5173(98)00338-X.
S. J. Byard, S. L. Jackson, A. Smail, M. Bauer, and D. C. Apperley. Studies on the crystallinity of a pharmaceutical development drug substance. J. Pharm. Sci. 94:1321–1335 (2005). doi:10.1002/jps.20328.
N. Kaneniwa, K. Imagawa, and M. Otsuka. Effect of tabletting on the degree of crystallinity and on the dehydration and decomposition points of cephalexin crystalline powder. Chem. Pharm. Bull. 33:802–809 (1985).
B. Shah, V. K. Kakumanu, and A. K. Bansal. Analytical Techniques for Quantification of Amorphous/Crystalline Phases in Pharmaceutical Solids. J. Pharm. Sci. 95:1641–1665 (2006). doi:10.1002/jps.20644.
G. Buckton, and P. Darcy. Assessment of disorder in crystalline powders—a review of analytical techniques and their application. Int. J. Pharm. 179:141–158 (1999). doi:10.1016/S0378-5173(98)00335-4.
X.-M. Zeng, G. P. Martin, C. Marriott, and J. Pritchard. Lactose as a carrier in dry powder formulations: the influence of surface characteristics on drug delivery. J. Pharm. Sci. 90:1424–1434 (2001). doi:10.1002/jps.1094.
I. Fix, and K.-J. Steffens. Quantifying low amorphous or crystalline amounts of alpha-lactose-monohydrate using X-ray powder diffraction, near infrared spectroscopy and differential scanning calorimetry. Drug Dev. Ind. Pharm. 30:513–523 (2004). doi:10.1081/DDC-120037482.
Á. Gombás, P. Szabó-Révész, M. Kata, G. Regdon Jr., and I. Erõs. Quantitative determination of crystallinity of a-lactose monohydrate by DSC. J. Therm. Anal. Calorim. 68:503–510 (2002). doi:10.1023/A:1016039819247.
M. Saunders, K. Podluii, S. Shergill, G. Buckton, and P. Royall. The potential of high speed DSC (Hyper-DSC) for the detection and quantification of small amounts of amorphous content in predominantly crystalline samples. Int. J. Pharm. 274:35–40 (2004). doi:10.1016/j.ijpharm.2004.01.018.
G. Buckton, P. Darcy, and A. J. Mackellar. The use of isothermal microcalorimetry in the study of small degrees of amorphous content of powders. Int. J. Pharm. 117:253–256 (1995). doi:10.1016/0378-5173(94)00421-Z.
K. Kawakami, T. Numa, and Y. Ida. Assessment of amorphous content by microcalorimetry. J. Pharm. Sci. 91:417–423 (2002). doi:10.1002/jps.10017.
S. E. Hogan, and G. Buckton. The quantification of small degrees of disorder in lactose using solution calorimetry. Int. J. Pharm. 207:57–64 (2000). doi:10.1016/S0378-5173(00)00527-5.
R. Lefort, A. De Gusseme, J.-F. Willart, F. Danede, and M. Descamps. Solid state NMR and DSC methods for quantifying the amorphous content in solid dosage forms: an application to ball-milling of trehalose. Int. J. Pharm. 280:209–219 (2004). doi:10.1016/j.ijpharm.2004.05.012.
J. W. Lubach, X. Dawei, B. E. Segmuller, and E. J. Munson. Investigation of the effects of pharmaceutical processing upon solid-state relaxation times and implications to solid-state formulation stability. J. Pharm. Sci. 96:777–787 (2006). doi:10.1002/jps.20684.
L. S. Taylor, and G. Zografi. The quantitative analysis of crystallinity using FT-Raman spectroscopy. Pharm. Res. 15:755–761 (1998). doi:10.1023/A:1011979221685.
P. Niemela, M. Paallysaho, P. Harjunen, M. Koivisto, V.-P. Lehto, J. Suhonen, and K. Jarvinen. Quantitative analysis of amorphous content of lactose using CCD-Raman spectroscopy. J. Pharm. Bio. Anal. 37:907–911 (2005). doi:10.1016/j.jpba.2004.09.027.
M. U. A. Ahlqvist, and L. S. Taylor. Water diffusion in hydrated crystalline and amorphous sugars monitored using H/D exchange. J. Pharm. Sci. 91:690–698 (2002). doi:10.1002/jps.10068.
M. U. A. Ahlqvist, and L. S. Taylor. Water dynamics in channel hydrates investigated using H/D exchange. Int. J. Pharm. 241:253–261 (2002). doi:10.1016/S0378-5173(02)00242-9.
J. Mann, and H. J. Marrinan. The reaction between cellulose and heavy water. 3. A quantitative study by infrared spectroscopy. Trans. Faraday Soc. 52:492–497 (1956). doi:10.1039/tf9565200492.
Y. Hishikawa, E. Togawa, Y. Kataoka, and T. Kondo. Characterization of amorphous domains in cellulosic materials using a FTIR deuteration monitoring analysis. Polymer. 40:7117–7124 (1999). doi:10.1016/S0032-3861(99)00120-2.
Y. Maréchal, and H. Chanzy. The hydrogen bond network in \({\text{I}}_\beta \) cellulose as observed by infrared spectrometry. J. Mol. Struct. 523:183–196 (2000). doi:10.1016/S0022-2860(99)00389-0.
E. Katainen, P. Niemelä, P. Harjunen, J. Suhonen, and K. Järvinen. Evaluation of the amorphous content of lactose by solution calorimetry and Raman spectroscopy. Talanta. 68:1–5 (2005). doi:10.1016/j.talanta.2005.04.062.
V.-P. Lehto, M. Tenho, K. Vähä-Heikkilä, P. Harjunen, M. Päällysaho, J. Välisaari, P. Niemelä, and K. Järvinen. The comparison of seven different methods to quantify the amorphous content of spray dried lactose. Powder Technol. 167:85–93 (2006). doi:10.1016/j.powtec.2006.05.019.
Á. Gombás, I. Antal, P. Szabó-Révész, S. Marton, and I. Erõs. Quantitative determination of crystallinity of alpha-lactose monohydrate by near infrared spectroscopy (NIRS). Int. J. Pharm. 256:25–32 (2003). doi:10.1016/S0378-5173(03)00059-0.
J. J. Seyer, P. E. Luner, and M. S. Kemper. Application of Diffuse Reflectance Near-infrared spectroscopy for determination of crystallinity. J. Pharm. Sci. 89:1305–1316 (2000). doi:10.1002/1520-6017(200010)89:10<1305::AID-JPS8>3.0.CO;2-Q.
M. V. Pellow-Jarman, P. J. Hendra, and R. J. Lenhart. The dependence of Raman signal intensity on particle size for crystal powders. Vib. Spectrosc. 12:257–261 (1996). doi:10.1016/0924-2031(96)00023-9.
D. J. Burnett, F. Thielmann, and J. Booth. Determining the critical relative humidity for moisture-induced phase transitions. Int. J. Pharm. 287:123–133 (2004). doi:10.1016/j.ijpharm.2004.09.009.
R. Young, and P. M. Price. Visualization of the crystallization of lactose from the amorphous state. J. Pharm. Sci. 93:155–164 (2004). doi:10.1002/jps.10549.
D. Lechuga-Ballesteros, A. Bakri, and D. P. Miller. Microcalorimetic measurement of the interactions between water vapor and amorphous pharmaceutical solids. Pharm. Res. 20:308–318 (2003). doi:10.1023/A:1022406709912.
Acknowledgements
The authors wish to thank the KTP for financial support of this project. Mr. Martin Dellar of the School of Chemistry, The University of Nottingham and Mr. John Fisher of Triton Technology are thanked for their assistance in the design and development of the dynamic deuteration apparatus. We are grateful to Mr. Andrew Camenisch and Ms. Abigail Shapland for their preliminary experimental work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Whiteside, P.T., Luk, S.Y., Madden-Smith, C.E. et al. Detection of Low Levels of Amorphous Lactose using H/D Exchange and FT-Raman Spectroscopy. Pharm Res 25, 2650–2656 (2008). https://doi.org/10.1007/s11095-008-9682-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9682-4