Abstract
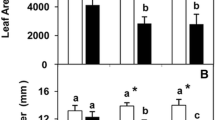
Plants grown in calcareous, high pH soils develop Fe deficiency chlorosis. While the physiological parameters of Fe-deficient leaves have been often investigated, there is a lack of information regarding structural leaf changes associated with such abiotic stress. Iron-sufficient and Fe-deficient pear and peach leaves have been studied, and differences concerning leaf epidermal and internal structure were found. Iron deficiency caused differences in the aspect of the leaf surface, which appeared less smooth in Fe-deficient than in Fe-sufficient leaves. Iron deficiency reduced the amount of soluble cuticular lipids in peach leaves, whereas it reduced the weight of the abaxial cuticle in pear leaves. In both plant species, epidermal cells were enlarged as compared to healthy leaves, whereas the size of guard cells was reduced. In chlorotic leaves, bundle sheaths were enlarged and appeared disorganized, while the mesophyll was more compacted and less porous than in green leaves. In contrast to healthy leaves, chlorotic leaves of both species showed a significant transient opening of stomata after leaf abscission (Iwanoff effect), which can be ascribed to changes found in epidermal and guard cells. Results indicate that Fe-deficiency may alter the barrier properties of the leaf surface, which can significantly affect leaf water relations, solute permeability and pest and disease resistance.




Similar content being viewed by others
References
Abadía J (1992) Leaf responses to Fe deficiency: a review. J Plant Nutr 15:1699–1713
Abadía A, Ambard-Bretteville F, Tremolières A (1988) Iron-deficiency in pea leaves: effect on lipid composition and synthesis. Physiol Plant 72:713–717
Álvarez-Fernández A, Grasa R, Abadía A, Sanz M, Abadía J (2003) Evaluación agronómica de nuevos quelatos de hierro. Phytoma 146:30–36
Álvarez-Fernández A, García-Laviña P, Fidalgo J, Abadía J, Abadía A (2004) Foliar fertilization to control iron chlorosis in pear (Pyrus communis L.) trees. Plant Soil 263:5–15
Álvarez-Fernández A, Abadía J, Abadía A (2006) Iron deficiency, fruit yield and quality. In: Abadía J, Barton LL (eds) Iron nutrition and interactions in plants. Springer, Dordrecht, pp 85–101
Anderson CA (1984) Development of leaf water deficits in detached green and lime-chlorotic leaves of seedlings from populations of Eucalyptus obliqua L’Hérit. Plant Soil 77:171–181
Bukovac MJ, Flore JA, Baker EA (1979) Peach leaf surfaces: changes in wettability, retention, cuticular permeability, and epicuticular wax chemistry during expansion with especial reference to spray application. J Am Soc Hortic Sci 104:611–617
Briat JF (2007) Iron dynamics in plants. In: Kader JC, Delseny M (eds) Advances in botanical research, vol 46: Incorporating advances in plant pathology. Academic, London, UK, pp 138–169. ISBN: 9780123737052
Conrad ME, Umbreit JN (2000) Iron absorption and transport—an update. Am J Hematol 64:287–298
DeMichele DW, Sharpe PJH (1973) An analysis of the mechanics of guard cell motion. J Theor Biol 41:77–96
Eichert T, Burkhardt J (2001) Quantification of stomatal uptake of ionic solutes using a new model system. J Exp Bot 52:771–781
Eichert T, Goldbach HE (2008) Equivalent pore radii of hydrophilic foliar uptake routes in stomatous and astomatous leaf surfaces—further evidence for a stomatal pathway. Physiol Plant 132:491–502
Eichert T, Goldbach HE, Burkhardt J (1998) Evidence for the uptake of large anions through stomatal pores. Bot Acta 111:461–466
Fernández V, Del Río V, Abadía J, Abadía A (2006) Foliar iron fertilization of peach (Prunus persica (L.) Batsch): effects of iron compounds, surfactants and other adjuvants. Plant Soil 289:239–252
Heredia A (2003) Biophysical and biochemical characteristics of cutin, plant biopolymer. Biochim Biophys Acta 1620:1–7
Holloway PJ (1969) The effects of surface wax on leaf wettability. Ann Appl Biol 63:145–153
Hutchinson TC (1970) Lime chlorosis as a factor in seedling establishment on calcareous soils. II. The development of leaf water deficits in plants showing lime-chlorosis. New Phytol 69:143–157
Iwanoff L (1928) Zur Methodik der Transpirationsbestimmung am Standort. Ber Dtsch Bot Ges 46:306–310
Jeffree CE (2006) The fine structure of the plant cuticle. In: Riederer M, Müller C (eds) Biology of the plant cuticle. Annual plant reviews, vol. 23. Blackwell, Oxford
Kaiser H, Legner N (2007) Localization of mechanisms involved in hydropassive and hydroactive stomatal responses of Sambucus nigra to dry air. Plant Physiol 143:1068–1077
Kerstiens G (2006) Water transport in plant cuticles: an update. J Exp Bot 57:2493–2499
Lange OL, Führer G, Gebel J (1986) Rapid field determination of photosynthetic capacity of cut spruce twigs (Picea abies) at saturation ambient CO2. Trees (Berl) 1:70–77
Larbi A, Morales F, Abadía J, Abadía A (2003) Effect of branch solid Fe implants on Fe xylem transport in peach and pear: changes in organic acid and Fe concentrations and pH. J Plant Physiol 160:1473–1482
Larbi A, Abadía A, Abadía J, Morales F (2006) Down co-regulation of light absorption, photochemistry, and carboxylation in Fe-deficient plants growing in different environments. Photosynth Res 89:113–126
Lucena JJ (2006) Synthetic iron chelates to correct iron deficiency in plants. In: Abadía J, Barton LL (eds) Iron nutrition and interactions in plants. Springer, Dordrecht, pp 103–128
Maldonado-Torres R, Etchevers-Barra JD, Alcántar-González G, Rodriguez-Alcazar J, Colinas-León MT (2006) Morphological changes in leaves of Mexican lime affected by iron chlorosis. J Plant Nutr 29:615–628
Monge E, Pérez C, Pequerul A, Madero P, Val J (1993) Effect of iron chlorosis on mineral nutrition and lipid composition of thylakoid biomembrane in Prunus persica (L.). Bastch Plant Soil 154:97–102
Morales F, Grasa R, Abadía A, Abadía J (1998) Iron chlorosis paradox in fruit trees. J Plant Nutr 21:815–825
Norris RF (1974) Penetration of 2,4-D in relation to cuticle thickness. Am J Bot 61:74–79
Norris RF, Bukovac MJ (1968) Structure of the pear leaf cuticle with special reference to cuticular penetration. Am J Bot 55:975–983
Powles JE, Buckley TN, Nicotra AB, Farquhar GC (2006) Dynamics of stomatal water relations following leaf excision. Plant Cell Environ 29:981–992
Raschke K (1970a) Leaf hydraulic system: rapid epidermal and stomatal responses to changes in water supply. Science 167:189–191
Raschke K (1970b) Stomatal responses to pressure changes and interruptions in the water supply of detached leaves of Zea mays L. Plant Physiol 45:415–423
Richardson A, Franke R, Kerstiens G, Jarvis M, Schreiber L, Fricke W (2005) Cuticular wax deposition in growing barley (Hordeum vulgare) leaves commences in relation to the point of emergence of epidermal cells from the sheaths of older leaves. Planta 222:472–483
Rombolà AD, Tagliavini M (2006) Iron nutrition of fruit tree crops. In: Abadía J, Barton LL (eds) Iron nutrition and interactions in plants. Springer, Dordrecht, The Netherlands, pp 61–83
Schmidt W (2003) Iron homeostasis in plants: sensing and signalling pathways. J Plant Nutr 26:2211–2230
Schönherr J, Riederer M (1988) Desorption of chemicals from plant cuticles: evidence for asymmetry. Arch Environ Contam Toxicol 17:13–19
Schreiber L (2006) Review of sorption and diffusion of lipophilic molecules in cuticular waxes and the effects of accelerators on solute mobilities. J Exp Bot 57:2515–2523
Shimshi D (1967) Leaf chlorosis and stomatal aperture. New Phytol 66:455–461
Terry N (1980) Limiting factors in photosynthesis. I. Use of iron stress to control photochemical capacity in vivo. Plant Physiol 65:114–129
Tichá I (1982) Photosynthetic characteristics during ontogenesis of leaves. 7. Stomata density and sizes. Photosynthetica 16:375–471
Turrell FM (1947) Citrus leaf stomata: structure, composition, and pore size in relation to penetration of liquids. Bot Gaz 108:476–483
Weyers JDB, Meidner H (1990) Methods in stomatal research. Longman Scientific & Technical, Essex, p 233
Acknowledgements
This study was supported by the Spanish Ministry of Science and Education (MEC, grants AGL2006-01416 and AGL2007-61948, co-financed with FEDER), the European Commission (ISAFRUIT project, Thematic Priority 5-Food Quality and Safety of the 6th Framework Programme of RTD; Contract no. FP6-FOOD-CT-2006-016279) and the Aragón Government (group A03). V.F. was supported by a “Juan de la Cierva”-MEC post-doctoral contract, co-financed by the European Social Fund. T.E. was supported by the CAI Europa XXI for a short term stay at the EEAD-CSIC. We would like to thank I. Tacchini and J.M. Andrés (ICB-CSIC, Zaragoza, Spain), F. Pinto (ICA-CSIC, Madrid, Spain) and R. Jordana (University of Navarra, Pamplona, Spain) for support with SEM techniques, L. Cistué for support with optical microscopy and image analysis. Thanks to Novozymes, for providing free sample products for experimental purposes.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ismail Cakmak.
Rights and permissions
About this article
Cite this article
Fernández, V., Eichert, T., Del Río, V. et al. Leaf structural changes associated with iron deficiency chlorosis in field-grown pear and peach: physiological implications. Plant Soil 311, 161–172 (2008). https://doi.org/10.1007/s11104-008-9667-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9667-4