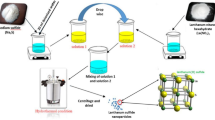
Abstract
In the present study, as-synthesized pure ammonium manganese phosphate hydrate (AMP) is infused with rGOx variate (Xmg = 25, 50, 75, 100) and four different hybrid composites (AMPG1, AMPG2, AMPG3 and AMPG4) have been synthesized by facile microwave route. The XRD results show two prominent peaks, one at 2θ = 10.04° (010) and other at 2θ = 31.3° (200) in all AMPGs. The Debye–Scherrer’s calculations show minimum crystallite size only for AMPG2 (80.9 nm). The Raman study confirms the rGO presence in AMPG2. The XPS confirms the existence of Mn as Mn2+ in AMPG2. The SEM/HR-TEM shows a cluster of uniform rectangular flake slabs only for AMPG2. The CV reveals that pure AMP and AMPGs exhibit pseudocapacitance. The GCD shows higher specific capacitance of 705 F g−1 at a current density of 1 A g−1 for AMPG2. The AMPG2//rGO hybrid device at 3 M aqueous H2SO4 shows higher specific capacitance of 336 F g−1 at 1 A g−1 in the potential window 0–1.8 volts, and even after 5000 cycles, the device retained 80% of its specific capacitance. The reason may be due to mapping of optimal concentration of rGO (50 mg) with \({\text{PO}}_{4}^{3 - }\) and \({\text{NH}}_{4}^{ + }\) of AMP by forming strong coordination for better activation sites for ion mobility. The energy and power densities of AMPG2//rGO device are 151 Wh kg−1 and 448 W kg−1 at 1 A g−1, which are reported for the first time for high-energy supercapacitor applications.
Graphic abstract














Similar content being viewed by others
References
Khan N, Dilshad S, Khalid R et al (2019) Review of energy storage and transportation of energy. Energy Storage 1:1–49. https://doi.org/10.1002/est2.49
Cho Y, Gabbar HA (2019) Review of energy storage technologies in harsh environment. Saf Extrem Environ 1:11–25. https://doi.org/10.1007/s42797-019-00002-9
Gür TM (2018) Review of electrical energy storage technologies, materials and systems: challenges and prospects for large-scale grid storage. Energy Environ Sci 11:2696–2767. https://doi.org/10.1039/c8ee01419a
Sufyan M, Rahim NA, Aman MM et al (2019) Sizing and applications of battery energy storage technologies in smart grid system: a review. J Renew Sustain Energy 11:014105. https://doi.org/10.1063/1.5063866
Koohi-Fayegh S, Rosen MA (2020) A review of energy storage types, applications and recent developments. J Energy Storage 27:101047. https://doi.org/10.1016/j.est.2019.101047
Kim BK, Sy S, Yu A, Zhang J (2014) Electrochemical supercapacitors for energy storage and conversion. Handb Clean Energy Syst. https://doi.org/10.1002/9781118991978.hces112
Wang F, Wu X, Yuan X et al (2017) Latest advances in supercapacitors: from new electrode materials to novel device designs. Chem Soc Rev 46:6816–6854. https://doi.org/10.1039/c7cs00205j
Krishnan SG, Reddy MV, Harilal M et al (2015) Characterization of MgCo2O4 as an electrode for high performance supercapacitors. Electrochim Acta 161:312–321. https://doi.org/10.1016/j.electacta.2015.02.081
Harilal M, Krishnan SG, Yar A, Misnon II, Reddy MV, Yusoff MM, Ojur Dennis J, Jose R (2017) Pseudocapacitive charge storage in single-step synthesized CoOMnO–MnCo O hybrid nanowires in aqueous alkaline electrolytes pseudocapacitive charge storage in single-step synthesized CoO–MnO2–MnCo2O4 hybrid nanowires in aqueous alkaline electrolyte. ACS J Phys Chem C 121:21171. https://doi.org/10.1021/acs.jpcc.7b06630
Reddy MV, Adams S, Barron AR et al (2017) Continuous nanobelts of nickel oxide–cobalt oxide hybrid with improved capacitive charge storage properties Midhun. Mater Des 122:376. https://doi.org/10.1016/j.matdes.2017.03.024
Vijayan BL, Misnon II, Anil GM, Kumar KM, Reddy MV, Zaghib K, Karuppaiah C, Yang C-C, Jose R (2019) Facile fabrication of thin metal oxide films on porous carbon for high density charge storage. J Colloid Interface Sci 562:567. https://doi.org/10.1016/j.jcis.2019.11.077
Vijayan BL, Khairiyyah N, Zain M et al (2020) Void space control in porous carbon for high density supercapacitive charge storage. Energy Fuels 34:5072. https://doi.org/10.1021/acs.energyfuels.0c00737
Najib S, Erdem E (2019) Current progress achieved in novel materials for supercapacitor electrodes: mini review. Nanoscale Adv 1:2817–2827. https://doi.org/10.1039/c9na00345b
Shao H, Padmanathan N, McNulty D et al (2016) Supercapattery based on binder-free Co3(PO4)2·8H2O multilayer nano/microflakes on nickel foam. ACS Appl Mater Interfaces 8:28592–28598. https://doi.org/10.1021/acsami.6b08354
Barzegar F, Momodu DY, Fashedemi OO, Bello A, Dangbegnon JK, Manyala N (2015) Investigation of different aqueous electrolytes on the electrochemical performance of activated carbon-based supercapacitors. RSC Adv 5:107482–107487. https://doi.org/10.1039/C5RA21962K
Qiu S, Xing W, Mu X et al (2016) A 3D nanostructure based on transition-metal phosphide decorated heteroatom-doped mesoporous nanospheres interconnected with graphene: synthesis and applications. ACS Appl Mater Interfaces 8:32528–32540. https://doi.org/10.1021/acsami.6b11101
Zhao H, Lan Y, Feng J et al (2018) Phosphorization boosts the capacitance of mixed metal nanosheet arrays for high performance supercapacitor electrodes. Nanoscale 10:11775–11781. https://doi.org/10.1039/c8nr01229f
Qing X, Cao Y, Wang J et al (2014) P/N/O co-doped carbonaceous material based supercapacitor with voltage up to 1.9 V in aqueous electrolyte. RSC Adv 4:55971–55979. https://doi.org/10.1039/c4ra06336h
Wen Y, Rufford TE, Hulicova-Jurcakova D, Wang L (2016) Nitrogen and phosphorous co-doped graphene monolith for supercapacitors. Chemsuschem 9:513–520. https://doi.org/10.1002/cssc.201501303
Zhao J, Pang H, Deng J et al (2013) Mesoporous uniform ammonium nickel phosphate hydrate nanostructures as high performance electrode materials for supercapacitors. CrystEngComm 15:5950–5955. https://doi.org/10.1039/c3ce40712h
Pang H, Yan Z, Wang W et al (2012) Template-free controlled fabrication of NH4MnPO4_H2O and Mn2P2O7 micro-nanostructures and study of their electrochemical properties. Int J Electrochem Sci 7:12340–12353
Pang H, Yan Z, Wang W et al (2012) Facile fabrication of NH4CoPO4·H2O nano/microstructures and their primarily application as electrochemical supercapacitor. Nanoscale 4:5946–5953. https://doi.org/10.1039/c2nr31208e
Liu J, Hu D, Huang T, Yu A (2012) Synthesis of flower-like LiMnPO4/C with precipitated NH4MnPO4·H2O as precursor. J Alloys Compd 518:58–62. https://doi.org/10.1016/j.jallcom.2011.12.134
Sharmila V, Parthibavarman M (2019) Facile synthesis of MnPO4·H2O nanosheets/MWCNTs composite as electrode material for high-performance supercapacitors. J Mater Sci: Mater Electron 30:19813–19825. https://doi.org/10.1007/s10854-019-02347-0
Luo P, Qiu Y, Guan X, Jiang L (2014) Regulation of photoluminescence properties of graphene quantum dots via hydrothermal treatment. Phys Chem Chem Phys 16:19011–19016. https://doi.org/10.1039/c4cp02652g
Xu J, Wei X, Cao J et al (2015) Facile synthesis and electrochemical performances of binder-free flexible graphene/acetylene black sandwich film. Electrochim Acta 152:391–397. https://doi.org/10.1016/j.electacta.2014.11.201
Park S, An J, Potts JR et al (2011) Hydrazine-reduction of graphite- and graphene oxide. Carbon N Y 49:3019–3023. https://doi.org/10.1016/j.carbon.2011.02.071
Reddy BJ, Vickraman P, Justin AS (2019) Microwave synthesis of MoO3-reduced graphene oxide nanocomposite for high performance asymmetric supercapacitors. J Mater Sci: Mater Electron 30:3618–3628. https://doi.org/10.1007/s10854-018-00641-x
Zhang Q, Qin Z, Luo Q et al (2017) Microstructure and nanoindentation behavior of Cu composites reinforced with graphene nanoplatelets by electroless co-deposition technique. Sci Rep 7:1338. https://doi.org/10.1038/s41598-017-01439-3
Lin L, Wang H, Xu P (2016) Immobilized TiO2-reduced graphene oxide nanocomposites on optical fibers as high performance photocatalysts for degradation of pharmaceuticals. Chem Eng J 310:389–398. https://doi.org/10.1016/j.cej.2016.04.024
Jayalakshmi M, Balasubramanian K (2008) Simple capacitors to supercapacitors: an overview. Int J Electrochem Sci 3:1196–1217
Xiong Z, Bin D, Zhang K et al (2016) Facile synthesis of MnPO4·H2O nanowire/graphene oxide composite material and its application as electrode material for high performance supercapacitors. Catalysts 6:198. https://doi.org/10.3390/catal6120198
El Khalfaouy R, Elabed A, Addaou A et al (2019) Synthesis and characterization of LiMnPO4 cathode material via dittmarite-type NH4MnPO4·H2O as an intermediate compound. Arab J Sci Eng 44:123–129. https://doi.org/10.1007/s13369-018-3248-5
Chen C, Zhang N, Liu X et al (2016) Polypyrrole-modified NH4NiPO4·H2O nanoplate arrays on Ni foam for efficient electrode in electrochemical capacitors. ACS Sustain Chem Eng 4:5578–5584. https://doi.org/10.1021/acssuschemeng.6b01347
Qiulin Z, Zhongyuan L, Zhenyu L et al (2012) Rapid synthesis of dittmarite by microwave-assisted hydrothermal method. Adv Mater Sci Eng 2012:1–4. https://doi.org/10.1155/2012/968396
Muhammad Hafiz S, Ritikos R, Whitcher TJ et al (2014) A practical carbon dioxide gas sensor using room-temperature hydrogen plasma reduced graphene oxide. Sens Actuators B Chem 193:692–700. https://doi.org/10.1016/j.snb.2013.12.017
Eda G, Chhowalla M (2010) Chemically derived graphene oxide: towards large-area thin-film electronics and optoelectronics. Adv Mater 22:2392–2415. https://doi.org/10.1002/adma.200903689
Pei S, Cheng HM (2012) The reduction of graphene oxide. Carbon N Y 50:3210–3228. https://doi.org/10.1016/j.carbon.2011.11.010
Sethi M, Bantawal H, Shenoy US, Bhat DK (2019) Eco-friendly synthesis of porous graphene and its utilization as high performance supercapacitor electrode material. J Alloys Compd 799:256–266. https://doi.org/10.1016/j.jallcom.2019.05.302
Srikanth VVSS, Venteshwara B, Chowdari R (2015) MgO decorated few-layered graphene as anode for Li ion batteries. ACS Appl Mater Interfaces 7:2301. https://doi.org/10.1021/am5064712
Johra FT, Jung WG (2015) Hydrothermally reduced graphene oxide as a supercapacitor. Appl Surf Sci 357:1911–1914. https://doi.org/10.1016/j.apsusc.2015.09.128
Kar P, Sardar S, Liu B et al (2016) Facile synthesis of reduced graphene oxide–gold nanohybrid for potential use in industrial waste-water treatment. Sci Technol Adv Mater 17:375–386. https://doi.org/10.1080/14686996.2016.1201413
Vittal JJ, Saravanan K, Reddy MV et al (2009) Storage performance of LiFePO4 nanoplates†. J Mater Chem 19:553–668. https://doi.org/10.1039/b817242k
Reddy MV, Rao GVS, Chowdari BVR (2010) Long-term cycling studies on 4 V-cathode, lithium vanadium fluorophosphate. J Power Sources 195:5768–5774. https://doi.org/10.1016/j.jpowsour.2010.03.032
Reddy MV, Yiming X, Rajarajan V et al (2015) Template free facile molten synthesis and energy storage studies on MCo2O4 (M = Mg, Mn) as anode for Li-ion batteries. ACS Sustain Chem Eng 3:3035–3042. https://doi.org/10.1021/acssuschemeng.5b00439
Nesbitt HW, Banerjee D (1998) Interpretation of XPS Mn(2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2 precipitation. Am Mineral 83:305–315. https://doi.org/10.2138/am-1998-3-414
Stranick M (1999) Mn2O3 by XPS. Surf Sci Spectra 6:39–46
Shimizu K, Shchukarev A (2011) X-ray photoelectron spectroscopy of fast-frozen hematite colloids in aqueous solutions. 3. Stabilization of ammonium species by surface (hydr) oxo groups. J Phys Chem 115:6796–6801
Terauchi T, Kobayashi Y, Iwai H, Tanaka A (2012) Protonic defect induced carrier doping in TTFCOO–NH4 + : tunable doping level by solvent. Synth Met 162:531–535. https://doi.org/10.1016/j.synthmet.2012.01.026
Chinnadurai D, Selvaraj AR, Rajendiran R et al (2018) Inhibition of redox behaviors in hierarchically structured manganese cobalt phosphate supercapacitor performance by surface trivalent cations. ACS Omega 3:1718–1725. https://doi.org/10.1021/acsomega.7b01762
Yang C, Dong L, Chen Z, Lu H (2014) High-performance all-solid-state supercapacitor based on the assembly of graphene and manganese(II) phosphate nanosheets. J Phys Chem C 118:18884–18891. https://doi.org/10.1021/jp504741u
Chen GZ (2013) Understanding supercapacitors based on nano-hybrid materials with interfacial conjugation. Prog Nat Sci Mater Int 23:245–255. https://doi.org/10.1016/j.pnsc.2013.04.001
Lee HY, Goodenough JB (1999) Supercapacitor behavior with KCl electrolyte. J Solid State Chem 144:220–223. https://doi.org/10.1006/jssc.1998.8128
Tang Y, Qiao Y, Zhao Y et al (2016) Hybridized phosphate with ultrathin nanoslices and single crystal microplatelets for high performance supercapacitors. Sci Rep 6:2–11. https://doi.org/10.1038/srep17613
Hao J, Li W, Zuo X et al (2019) Facile electrochemical phosphatization of Mn3O4 nanosheet arrays for supercapacitor with enhanced performance. J Mater Sci 54:625–637. https://doi.org/10.1007/s10853-018-2842-y
Reddy BJ, Vickraman P, Justin AS (2019) Asymmetric supercapacitor device performance based on microwave synthesis of N-doped graphene/nickel sulfide nanocomposite. J Mater Sci 54:6361–6373. https://doi.org/10.1007/s10853-018-03314-6
Bai Y, Du M, Chang J et al (2014) Supercapacitors with high capacitance based on reduced graphene oxide/carbon nanotubes/NiO composite electrodes†. J Mater Chem A 2:3834–3840. https://doi.org/10.1039/c3ta15004f
Liu Y, Zhai X, Yang K et al (2019) Mesoporous NH4 NiPO4 ·H2O for high-performance flexible all-solid-state asymmetric supercapacitors. Front Chem. https://doi.org/10.3389/fchem.2019.00118
Arul Raja T, Vickraman P, Simon Justin A, Joji Reddy B (2019) Microwave synthesis of zinc ammonium phosphate/reduced graphene oxide hybrid composite for high energy density supercapacitors. Phys Status Solidi Appl Mater Sci 217:1900736. https://doi.org/10.1002/pssa.201900736
Yan K, Kang L, Dai Y-H et al (2016) Facile fabrication of manganese phosphate nanosheets for supercapacitor applications. Ionics (Kiel) 22:1461–1469. https://doi.org/10.1007/s11581-016-1652-y
Numan A, Ramesh K, Lee CC et al (2017) An enhanced performance of hybrid supercapacitor based on polyaniline-manganese phosphate binary composite. J Solid State Electrochem 21:3205–3213. https://doi.org/10.1007/s10008-017-3624-1
Priyadharsini N, Shanmugavani A, Vasylechko L, Kalai Selvan R (2018) Sol-gel synthesis, structural refinement, and electrochemical properties of potassium manganese phosphate for supercapacitors. Ionics (Kiel) 24:2073–2082. https://doi.org/10.1007/s11581-018-2449-y
Madito MJ, Oyedotun KO, Masikhwa TM et al (2017) Hydrothermal synthesis of manganese phosphate/graphene foam composite for electrochemical supercapacitor applications. J Colloid Interface Sci 494:325–337. https://doi.org/10.1016/j.jcis.2017.01.098
Kai-Bing Li, Da-Wei Shi, Zhi-Yong Cai, Guo-Liang Z, Qiu-An Huang LDC-P (2015) Studies on the equivalent serial resistance of carbon supercapacitor. Electrochim Acta. https://doi.org/10.1016/j.electacta.2015.06.008
Wang P, Wang T, Lin W et al (2018) Enhanced supercapacitor performance using electropolymerization of self-doped polyaniline on carbon film. Nanomaterials 8:214. https://doi.org/10.3390/nano8040214
Guo Yanni, He Deliang, Xie Aomei, Wei Qu, Yining Tang LZ, Zhu R (2019) The electrochemical oxidation of hydroquinone and catechol through a novel poly-geminal dicationic ionic liquid (PGDIL)–TiO2 composite film electrode. Polymers (Basel) 11:1907
Jain D, Kanungo J (2018) Enhanced performance of ultracapacitors using redox additive-based electrolytes. Appl Phys A 124:14. https://doi.org/10.1007/s00339-018-1814-z
Ma W, Xie L, Dai L et al (2018) Influence of phosphorus doping on surface chemistry and capacitive behaviors of porous carbon electrode. Electrochim Acta 266:420–430. https://doi.org/10.1016/j.electacta.2018.02.031
Sarkar A, Gopal G (2018) Synthesis of BiFeO3 nanoparticle anchored TiO2-BiFeO3 nanoheterostructure and exploring its different electrochemical aspects as electrode. Mater Today Proc 5:10177–10184. https://doi.org/10.1016/j.matpr.2017.11.016
Liu R, Cho SI, Lee SB (2008) Poly(3,4-ethylenedioxythiophene) nanotubes as electrode materials for a high-powered supercapacitor. Nanotechnology 19:215710. https://doi.org/10.1088/0957-4484/19/21/215710
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors hereby declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raja, T., Vickraman, P., Justin, A.S. et al. Electrochemical studies on NH4MnPO4.H2O–rGO Hybrid Composite Synthesized via Microwave Route for High Energy Supercapacitors. J Mater Sci 55, 14447–14463 (2020). https://doi.org/10.1007/s10853-020-05032-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05032-4