Abstract
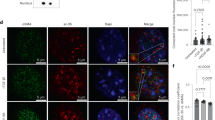
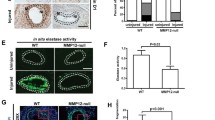
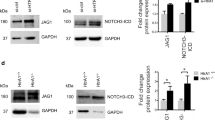
Heparan sulfate proteoglycans act as co-receptors for many chemokines and growth factors. The sulfation pattern of the heparan sulfate chains is a critical regulatory step affecting the binding of chemokines and growth factors. N-deacetylase-N-sulfotransferase1 (Ndst1) is one of the first enzymes to catalyze sulfation. Previously published work has shown that HSPGs alter tangent moduli and stiffness of tissues and cells. We hypothesized that loss of Ndst1 in smooth muscle would lead to significant changes in heparan sulfate modification and the elastic properties of arteries. In line with this hypothesis, the axial tangent modulus was significantly decreased in aorta from mice lacking Ndst1 in smooth muscle (SM22αcre+Ndst1−/−, p < 0.05, n = 5). The decrease in axial tangent modulus was associated with a significant switch in myosin and actin types and isoforms expressed in aorta and isolated aortic vascular smooth muscle cells. In contrast, no changes were found in the compliance of smaller thoracodorsal arteries of SM22αcre+Ndst1−/− mice. In summary, the major findings of this study were that targeted ablation of Ndst1 in smooth muscle cells results in altered biomechanical properties of aorta and differential expression of myosin and actin types and isoforms.




Similar content being viewed by others
References
Esko JD, Selleck SB (2002) Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem 71:435–471. doi:10.1146/annurev.biochem.71.110601.135458
Kim S-H, Turnbull J, Guimond S (2011) Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 209:139–151. doi:10.1530/joe-10-0377
Kirkpatrick CA, Selleck SB (2007) Heparan sulfate proteoglycans at a glance. J Cell Sci 120:1829–1832. doi:10.1242/jcs.03432
Lander AD, Selleck SB (2000) The elusive functions of proteoglycans: in vivo veritas. J Cell Biol 148:227–232. doi:10.1083/jcb.148.2.227
Sarrazin S, Lamanna WC, Esko JD (2011) Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. doi:10.1101/cshperspect.a004952
Selleck SB (1998) Genetic analysis of functions for cell surface proteoglycans. Matrix Biol 17:473–476. doi:10.1016/S0945-053X(98)90094-4
Selleck SB (2000) Proteoglycans and pattern formation: sugar biochemistry meets developmental genetics. Trends Genet 16:206–212. doi:10.1016/S0168-9525(00)01997-1
Sheng J, Liu R, Xu Y, Liu J (2011) The dominating role of N-deacetylase/N-sulfotransferase 1 in forming domain structures in heparan sulfate. J Biol Chem 286:19768–19776. doi:10.1074/jbc.M111.224311
Wight TN, Merrilees MJ (2004) Proteoglycans in atherosclerosis and restenosis. Circ Res 94:1158–1167. doi:10.1161/01.res.0000126921.29919.51
Takahashi R, Negishi K, Watanabe A, Arai M, Naganuma F, Ohyama Y, Kurabayashi M (2011) Serum syndecan-4 is a novel biomarker for patients with chronic heart failure. J Cardiol 57:325–332. doi:10.1016/j.jjcc.2011.01.012
Vanhoutte D, Schellings MWM, Götte M, Swinnen M, Herias V et al (2007) Increased expression of syndecan-1 protects against cardiac dilatation and dysfunction after myocardial infarction. Circulation 115:475–482. doi:10.1161/circulationaha.106.644609
Tannock L, King V (2008) Proteoglycan mediated lipoprotein retention: a mechanism of diabetic atherosclerosis. Rev Endocr Metab Disord 9:289–300. doi:10.1007/s11154-008-9078-0
Wade A, Robinson AE, Engler JR, Petritsch C, James CD, Phillips JJ (2013) Proteoglycans and their roles in brain cancer. FEBS J 280:2399–2417. doi:10.1007/s11154-008-9078-0
Cotman SL, Halfter W, Cole GJ (2000) Agrin binds to β-amyloid (Aβ), accelerates Aβ fibril formation, and is localized to Aβ deposits in Alzheimer’s disease brain. Mol Cell Neurosci 15:183–198. doi:10.1006/mcne.1999.0816
Chirinos J (2012) Arterial stiffness: basic concepts and measurement techniques. J Cardiovasc Transl Res 5:243–255. doi:10.1007/s12265-012-9359-6
Duprez DA, Cohn JN (2007) Arterial stiffness as a risk factor for coronary atherosclerosis. Curr Atheroscler Rep 9:139–144. doi:10.1007/s11883-007-0010-y
Balasubramani M, Schreiber EM, Candiello J, Balasubramani GK, Kurtz J, Halfter W (2010) Molecular interactions in the retinal basement membrane system: a proteomic approach. Matrix Biol 29:471–483. doi:10.1016/j.matbio.2010.04.002
Buczek-Thomas JA, Chu CL, Rich CB, Stone PJ, Foster JA, Nugent MA (2002) Heparan sulfate depletion within pulmonary fibroblasts: implications for elastogenesis and repair. J Cell Physiol 192:294–303. doi:10.1002/jcp.10135
Cluff A, Bystrom B, Klimaviciute A, Dahlqvist C, Cebers G, Malmstrom A, Ekman-Ordeberg G (2006) Prolonged labour associated with lower expression of syndecan 3 and connexin 43 in human uterine tissue. Reprod Biol Endocrinol 4:24. doi:10.1186/1477-7827-4-24
Elenius V, Götte M, Reizes O, Elenius K, Bernfield M (2004) Inhibition by the soluble syndecan-1 ectodomains delays wound repair in mice overexpressing syndecan-1. J Biol Chem 279:41928–41935. doi:10.1074/jbc.M404506200
Feldhammer M, Durand S, Mrázová L, Boucher R-M, Laframboise R et al (2009) Sanfilippo syndrome type C: mutation spectrum in the heparan sulfate acetyl-CoA: α-glucosaminide N-acetyltransferase (HGSNAT) gene. Hum Mutat 30:918–925. doi:10.1002/humu.20986
Gambillara V, Thacher T, Silacci P, Stergiopulos N (2008) Effects of reduced cyclic stretch on vascular smooth muscle cell function of pig carotids perfused ex vivo. Am J Hypertens 21:425–431. doi:10.1038/ajh.2007.72
Gheduzzi D, Guerra D, Bochicchio B, Pepe A, Tamburro AM et al (2005) Heparan sulphate interacts with tropoelastin, with some tropoelastin peptides and is present in human dermis elastic fibers. Matrix Biol 24:15–25. doi:10.1016/j.matbio.2004.12.001
Götte M, Spillmann D, Yip GW, Versteeg E, Echtermeyer FG, van Kuppevelt TH, Kiesel L (2008) Changes in heparan sulfate are associated with delayed wound repair, altered cell migration, adhesion and contractility in the galactosyltransferase I (ß4GalT-7) deficient form of Ehlers–Danlos syndrome. Hum Mol Genet 17:996–1009. doi:10.1093/hmg/ddm372
Hocking DC, Kowalski K (2002) A cryptic fragment from fibronectin’s III1 module localizes to lipid rafts and stimulates cell growth and contractility. J Cell Biol 158:175–184. doi:10.1083/jcb.200112031
Hultgårdh-Nilsson A, Durbeej M (2007) Role of the extracellular matrix and its receptors in smooth muscle cell function: implications in vascular development and disease. Curr Opin Lipidol 18:540–545. doi:10.1097/MOL.0b013e3282ef77e9
Huveneers S, Truong H, Fässler R, Sonnenberg A, Danen EHJ (2008) Binding of soluble fibronectin to integrin α5β1—link to focal adhesion redistribution and contractile shape. J Cell Sci 121:2452–2462. doi:10.1242/jcs.033001
Isnard N, Fodil-Bourahla I, Robert AM, Robert L (2004) Pharmacology of skin aging. Stimulation of glycosaminoglycan biosynthesis by l-fucose and fucose-rich polysaccharides, effect of in vitro aging of fibroblasts. Biomed Pharmacother 58:202–204. doi:10.1016/j.biopha.2003.07.002
Jimenez-Vergara AC, Munoz-Pinto DJ, Becerra-Bayona S, Wang B, Iacob A, Hahn MS (2011) Influence of glycosaminoglycan identity on vocal fold fibroblast behavior. Acta Biomater 7:3964–3972. doi:10.1016/j.actbio.2011.06.034
Marucha J, Tylki-Szymańska A, Jakóbkiewicz-Banecka J, Piotrowska E, Kloska A, Czartoryska B, Węgrzyn G (2011) Improvement in the range of joint motion in seven patients with mucopolysaccharidosis type II during experimental gene expression-targeted isoflavone therapy (GET IT). Am J Med Genet A 155:2257–2262. doi:10.1002/ajmg.a.34146
Negrini D, Tenstad O, Passi A, Wiig H (2006) Differential degradation of matrix proteoglycans and edema development in rabbit lung. Am J Physiol 290:L470–L477. doi:10.1152/ajplung.0.0310.2005
Nemes A, Timmermans RM, Wilson JHP, Soliman OI, Krenning B, Cate FJ, Geleijnse ML (2008) The mild form of mucopolysaccharidosis type I (Scheie syndrome) is associated with increased ascending aortic stiffness. Heart Vessels 23:108–111. doi:10.1007/s00380-007-1013-x
O’Callaghan R, Job KM, Dull RO, Hlady V (2011) Stiffness and heterogeneity of the pulmonary endothelial glycocalyx measured by atomic force microscopy. Am J Physiol 301:L353–L360. doi:10.1152/ajplung.0.0342.2010
Okina E, Manon-Jensen T, Whiteford JR, Couchman JR (2009) Syndecan proteoglycan contributions to cytoskeletal organization and contractility. Scand J Med Sci Sports 19:479–489. doi:10.1111/j.1600-0838.2009.00941.x
Schwinn MK, Gonzalez JM Jr, Gabelt BAT, Sheibani N, Kaufman PL, Peters DM (2010) Heparin II domain of fibronectin mediates contractility through an α4β1 co-signaling pathway. Exp Cell Res 316:1500–1512. doi:10.1016/j.yexcr.2010.03.010
Stum M, Girard E, Bangratz M, Bernard V, Herbin M et al (2008) Evidence of a dosage effect and a physiological endplate acetylcholinesterase deficiency in the first mouse models mimicking Schwartz–Jampel syndrome neuromyotonia. Hum Mol Genet 17:3166–3179. doi:10.1093/hmg/ddn213
Wilusz RE, DeFrate LE, Guilak F (2012) A biomechanical role for perlecan in the pericellular matrix of articular cartilage. Matrix Biol 31:320–327. doi:10.1016/j.matbio.2012.05.002
Yang JJ, Chen YM, Kurokawa T, Gong JP, Onodera S, Onodera S, Yasuda K (2010) Gene expression, glycocalyx assay, and surface properties of human endothelial cells cultured on hydrogel matrix with sulfonic moiety: effect of elasticity of hydrogel. J Biomed Mater Res A 95A:531–542. doi:10.1002/jbm.a.32875
Shi Z-D, Abraham G, Tarbell JM (2010) Shear stress modulation of smooth muscle cell marker genes in 2-D and 3-D depends on mechanotransduction by heparan sulfate proteoglycans and ERK1/2. PLoS ONE 5:e12196. doi:10.1371/journal.pone.0012196
Adhikari N, Rusch M, Li Q, Selleck SB, Hall JL (2004) Significant alterations in heparan sulfate biosynthesis in response to vascular injury. Circulation 11:III-335
Bingley JA, Hayward IP, Campbell JH, Campbell GR (1998) Arterial heparan sulfate proteoglycans inhibit vascular smooth muscle cell proliferation and phenotype change in vitro and neointimal formation in vivo. J Vasc Surg 28:308–318. doi:10.1016/S0741-5214(98)70167-3
Segev A, Nili N, Strauss BH (2004) The role of perlecan in arterial injury and angiogenesis. Cardiovasc Res 63:603–610. doi:10.1016/j.cardiores.2004.03.028
Theocharis AD, Theocharis DA, De Luca G, Hjerpe A, Karamanos NK (2002) Compositional and structural alterations of chondroitin and dermatan sulfates during the progression of atherosclerosis and aneurysmal dilatation of the human abdominal aorta. Biochimie 84:667–674. doi:10.1016/S0300-9084(02)01428-1
Tovar AMF, Teixeira LAC, Marinho ACO, Pinho DA, Silva L-F, Mourão PAS (2011) The dermatan sulfate-dependent anticoagulant pathway is mostly preserved in aneurysm and in severe atherosclerotic lesions while the heparan sulfate pathway is disrupted. Clin Chim Acta 412:906–913. doi:10.1016/j.cca.2011.01.016
Tran PK, Tran-Lundmark K, Soininen R, Tryggvason K, Thyberg J, Hedin U (2004) Increased intimal hyperplasia and smooth muscle cell proliferation in transgenic mice with heparan sulfate-deficient perlecan. Circ Res 94:550–558. doi:10.1161/01.RES.0000117772.86853.34
Tran-Lundmark K, Tran P-K, Paulsson-Berne G, Friden V, Soininen R et al (2008) Heparan sulfate in perlecan promotes mouse atherosclerosis: roles in lipid permeability, lipid retention, and smooth muscle cell proliferation. Circ Res 103:43–52. doi:10.1161/CIRCRESAHA.108.172833
Adhikari N, Basi DL, Townsend D, Rusch M, Mariash A, Mullegama S, Watson A, Larson J et al (2010) Heparan sulfate Ndst1 regulates vascular smooth muscle cell proliferation, vessel size and vascular remodeling. J Mol Cell Cardiol 49:287–293. doi:10.1016/j.yjmcc.2010.02.022
Barbosa-Morais NL, Dunning MJ, Samarajiwa SA, Darot JFJ, Ritchie ME, Lynch AG, Tavaré S (2010) A re-annotation pipeline for Illumina BeadArrays: improving the interpretation of gene expression data. Nucleic Acids Res 38:e17. doi:10.1093/nar/gkp942
Ray JL, Leach R, Herbert JM, Benson M (2001) Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci 23:185–188. doi:10.1023/A:1016357510143
Adhikari N, Basi DL, Carlson M, Mariash A, Hong Z et al (2011) Increase in GLUT1 in smooth muscle alters vascular contractility and increases inflammation in response to vascular injury. Arterioscler Thromb Vasc Biol 31:86–94. doi:10.1161/atvbaha.110.215004
Billaud M, Lohman AW, Straub AC, Parpaite T, Johnstone SR, Isakson BE (2012) Characterization of the thoracodorsal artery: morphology and reactivity. Microcirculation 19:360–372. doi:10.1111/j.1549-8719.2012.00172.x
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B 57:289–300. doi:10.2307/2346101
Montaniel Kim Ramil C, Billuad M, Graham C, Kim SK, Carlson M, Zeng W, Zeng O, Pan W et al (2012) Smooth muscle specific deletion of Ndst1 leads to decreased vessel luminal area and no change in blood pressure in conscious mice. J Cardiovasc Transl Res 5:274–279. doi:10.1007/s12265-012-9369-4
Agianniotis A, Stergiopulos N (2012) Wall properties of the apolipoprotein E-deficient mouse aorta. Atherosclerosis 223:314–320. doi:10.1016/j.atherosclerosis.2012.06.014
Eberth JF, Popovic N, Gresham VC, Wilson E, Humphrey JD (2010) Time course of carotid artery growth and remodeling in response to altered pulsatility. Am J Physiol 299:H1875–H1883. doi:10.1152/ajpheart.0.0872.2009
Hayenga HN, Trache A, Trzeciakowski J, Humphrey JD (2011) Regional atherosclerotic plaque properties in ApoE−/− mice quantified by atomic force, immunofluorescence, and light microscopy. J Vasc Res 48:495–504. doi:10.1159/000329586
Frid MG, Aldashev AA, Dempsey EC, Stenmark KR (1997) Smooth muscle cells isolated from discrete compartments of the mature vascular media exhibit unique phenotypes and distinct growth capabilities. Circ Res 81:940–952. doi:10.1161/01.res.81.6.940
Hao H, Ropraz P, Verin V, Camenzind E, Geinoz A, Pepper MS, Gabbiani G, Bochaton-Piallat M-L (2002) Heterogeneity of smooth muscle cell populations cultured from pig coronary artery. Arterioscler Thromb Vasc Biol 22:1093–1099. doi:10.1161/01.atv.0000022407.91111.e4
Koyama N, Kinsella M, Wight TN, Hedin U, Clowes AW (1998) Heparan sulfate proteoglycans mediate a potent inhibitory signal for migration of vascular smooth muscle cells. Circ Res 83:305–313. doi:10.1161/01.res.83.3.305
Godman CA, Chheda KP, Hightower LE, Perdrizet G, Shin D-G, Giardina C (2010) Hyperbaric oxygen induces a cytoprotective and angiogenic response in human microvascular endothelial cells. Cell Stress Chaperones 15:431–442. doi:10.1007/s12192-009-0159-0
Lachaud CC, Pezzolla D, Domínguez-Rodríguez A, Smani T, Soria B, Hmadcha A (2013) Functional vascular smooth muscle-like cells derived from adult mouse uterine mesothelial cells. PLoS ONE 8:e55181. doi:10.1371/journal.pone.0055181
Zielinska M, Sawosz E, Grodzik M, Wierzbicki M, Gromadka M et al (2011) Effect of heparan sulfate and gold nanoparticles on muscle development during embryogenesis. Int J Nanomed 6:3163–3172. doi:10.2147/Ijn.S26070
Mohamed JS, Boriek AM (2012) Loss of desmin triggers mechanosensitivity and up-regulation of Ankrd1 expression through Akt-NF-κB signaling pathway in smooth muscle cells. FASEB J 26:757–765. doi:10.1096/fj.10-160291
Hu G, Zhou R, Liu J, Gong A-Y, Eischeid AN, Dittman JW, Chen X-M (2009) MicroRNA-98 and let-7 confer cholangiocyte expression of cytokine-inducible Src homology 2-containing protein in response to microbial challenge. J Immunol 183:1617–1624. doi:10.4049/jimmunol.0804362
Khor CC, Vannberg FO, Chapman SJ, Guo H, Wong SH et al (2010) CISH and susceptibility to infectious diseases. N Engl J Med 362:2092–2101. doi:10.1056/NEJMoa0905606
Ram PA, Waxman DJ (1999) SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J Biol Chem 274:35553–35561
Karlsson-Lindahl L, Schmidt L, Haage D, Hansson C, Taube M et al (2012) Heparanase affects food intake and regulates energy balance in mice. PLoS ONE 7:e34313. doi:10.1074/jbc.274.50.35553
Ren Y, Kirkpatrick CA, Rawson JM, Sun M, Selleck SB (2009) Cell type-specific requirements for heparan sulfate biosynthesis at the Drosophila neuromuscular junction: effects on synapse function, membrane trafficking, and mitochondrial localization. J Neurosci 29:8539–8550. doi:10.1523/jneurosci.5587-08.2009
Sarparanta J (2008) Biology of myospryn: what’s known? J Muscle Res Cell Motil 29:177–180. doi:10.1007/s10974-008-9165-6
Kouloumenta A, Mavroidis M, Capetanaki Y (2007) Proper perinuclear localization of the TRIM-like protein myospryn requires its binding partner desmin. J Biol Chem 282:35211–35221. doi:10.1074/jbc.M704733200
Knöll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I et al (2002) The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 111:943–955. doi:10.1016/S0092-8674(02)01226-6
Wang X, Li Q, Adhikari N, Hall JL (2006) A role for muscle LIM protein (MLP) in vascular remodeling. J Mol Cell Cardiol 40:503–509. doi:10.1016/j.yjmcc.2006.01.005
Amatschek S, Lucas R, Eger A, Pflueger M, Hundsberger H et al (2011) CXCL9 induces chemotaxis, chemorepulsion and endothelial barrier disruption through CXCR3-mediated activation of melanoma cells. Br J Cancer 469–479. doi:10.1038/sj.bjc.6606056
Lu X, Wang L, Chen S, He L, Yang X et al (2012) Genome-wide association study in Han Chinese identifies four new susceptibility loci for coronary artery disease. Nat Genet 44:890–894. doi:10.1038/ng.2337
The International Consortium for Blood Pressure Genome-Wide Association Studies (2011) Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478:103–109. doi:10.1038/nature10405
Schudt C, Hatzelmann A, Beume R, Tenor H (2011) Phosphodiesterase inhibitors: history of pharmacology. In: Francis SH, Conti M, Houslay MD (eds) Phosphodiesterases as drug targets. Springer, Berlin, pp 1–46. doi:10.1007/978-3-642-17969-3_1
Withrington PG, Dhume VG, Croxton R, Gerbes AL (1990) The actions of human atrial natriuretic factor on hepatic arterial and portal vascular beds of the anaesthetized dog. Br J Pharmacol 99:810–814. doi:10.1111/j.1476-5381.1990.tb13011.x
Echaniz-Laguna A, Rene F, Marcel C, Bangratz M, Fontaine B, Loeffler J-P, Nicole S (2009) Electrophysiological studies in a mouse model of Schwartz–Jampel syndrome demonstrate muscle fiber hyperactivity of peripheral nerve origin. Muscle Nerve 40:55–61. doi:10.1111/j.1476-5381.1990.tb13011.x
Acknowledgments
This study was funded by a R01 to JLH (National Institute of Health-R01HL081715). Special thanks to the staff of the Histology Core facility, Lillehei Heart Institute, and Cynthia Dekay, Graphic Designer, Lillehei Heart Institute, for their help in the preparation of this manuscript.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Adhikari, N., Billaud, M., Carlson, M. et al. Vascular biomechanical properties in mice with smooth muscle specific deletion of Ndst1. Mol Cell Biochem 385, 225–238 (2014). https://doi.org/10.1007/s11010-013-1831-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1831-3