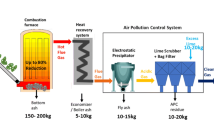
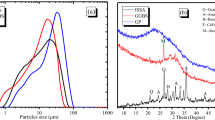
Abstract
Solid waste production is rapidly increasing, and municipal solid waste incinerator plants provide a practical and sustainable solution to significantly reduce the volume of waste. Incinerator ash is a byproduct of the combustion process, and lightweight sands can be reclaimed from the ash for use in cementitious materials once treated for hydrogen gas production. This work investigates treatment methods for reclaimed sands for use in concrete. A novel method based on a propriety patent to capture the amount of hydrogen gas production from reclaimed sands is presented using a steel pressure chamber, pressure transducer, and data acquisition system. The setup is maintained under a constant temperature, pressure, and agitation using an environmental incubator. Treatment methods using sodium hydroxide, reused sodium hydroxide, alumina, and alumina + sodium hydroxide are investigated. It is found that sodium hydroxide is an effective treatment solution for reclaimed sands, with the ability to reuse the solution multiple times. Alumina is found not to be an effective treatment method when used alone. Concrete is made using treated reclaimed sands, where it is shown through scanning electron microscopy imaging that large voids in the cement matrix due to hydrogen gas production are significantly reduced in size.





Similar content being viewed by others
References
Kim J, Nam BH, Al Muhit BA, Tasneem KM, An J (2015) Effect of chemical treatment of MSWI bottom ash for its use in concrete. Mag Concr Res 67:179–186. https://doi.org/10.1680/macr.14.00170
Bertolini L, Carsana M, Cassago D, Quadrio Curzio A, Collepardi M (2004) MSWI ashes as mineral additions in concrete. Cement Concr Res 34:1899–1906. https://doi.org/10.1016/J.CEMCONRES.2004.02.001
Van Praagh M, Johansson M, Fagerqvist J, Grönholm R, Hansson N, Svensson H (2018) Recycling of MSWI-bottom ash in paved constructions in Sweden—a risk assessment. Waste Manag 79:428–434. https://doi.org/10.1016/J.WASMAN.2018.07.025
Lynn CJ, Ghataora GS, Dhir OBERK (2017) Municipal incinerated bottom ash (MIBA) characteristics and potential for use in road pavements. Int J Pavement Res Technol 10:185–201. https://doi.org/10.1016/J.IJPRT.2016.12.003
Holmes N, O’Malley H, Cribbin P, Mullen H, Keane G (2016) Performance of masonry blocks containing different proportions of incinator bottom ash. Sustain Mater Technol 8:14–19. https://doi.org/10.1016/J.SUSMAT.2016.05.001
Mathews IV G, Soliman M, Dalesandro K, Young M (2019) Performance of reclaimed waste to energy aggregates as lightweight sand in concrete masonry units. In: 13th North American Masonry Conference. Salt Lake City
Tang P, Florea MVA, Spiesz P, Brouwers HJH (2015) Characteristics and application potential of municipal solid waste incineration (MSWI) bottom ashes from two waste-to-energy plants. Constr Build Mater 83:77–94. https://doi.org/10.1016/J.CONBUILDMAT.2015.02.033
Li X, Liu Z, Lv Y, Cai L, Jiang D, Jiang W et al (2018) Utilization of municipal solid waste incineration bottom ash in autoclaved aerated concrete. Constr Build Mater 178:175–182. https://doi.org/10.1016/J.CONBUILDMAT.2018.05.147
Mathews G, Sinnan R, Young M (2019) Evaluation of reclaimed municipal solid waste incinerator sands in concrete. J Clean Prod 229:838–849. https://doi.org/10.1016/J.JCLEPRO.2019.04.387
Xia Y, He P, Shao L, Zhang H (2017) Metal distribution characteristic of MSWI bottom ash in view of metal recovery. J Environ Sci 52:178–189. https://doi.org/10.1016/J.JES.2016.04.016
Petrovic J, Thomas G (2008) Reaction of aluminum with water to produce hydrogen: a study of issues related to the use of aluminum for on-board vehicular hydrogen storage. U.S. Department of Energy Report. https://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/aluminium_water_hydrogen.pdf
Ichikawa T, Yamada K, Osako M, Haga K (2017) Suppression of hydrogen gas evolution from cement-solidified MSWI fly ash. J Adv Concr Technol 15:574–578
Xuan D, Poon CS (2018) Removal of metallic Al and Al/Zn alloys in MSWI bottom ash by alkaline treatment. J Hazard Mater 344:73–80. https://doi.org/10.1016/J.JHAZMAT.2017.10.002
Nithiya A, Saffarzadeh A, Shimaoka T (2018) Hydrogen gas generation from metal aluminum-water interaction in municipal solid waste incineration (MSWI) bottom ash. Waste Manag 73:342–350. https://doi.org/10.1016/J.WASMAN.2017.06.030
Verbinnen B, Billen P, Van Caneghem J, Vandecasteele C (2017) Recycling of MSWI bottom ash: a review of chemical barriers, engineering applications and treatment technologies. Waste Biomass Valoriz 8:1453–1466. https://doi.org/10.1007/s12649-016-9704-0
Joseph AM, Van den Heeded P, Snellings R, Van A (2017) Comparison of different beneficiation techniques to improve utilization potential of municpal solid waste incineration fly ash concrete. In: Constr. Mater. Syst., pp 49–54
Hiraki T, Takeuchi M, Hisa M, Akiyama T (2005) Hydrogn production from waste aluminum at different temperatures with LCA. Mater Trans 45:1052–1057
Belitskus D (1970) Reaction of aluminum with sodium hydroxide solution as a source of hydrogen. J Electrochem Soc 117:1097. https://doi.org/10.1149/1.2407730
Anderson ER, Andersen EJ (2003) Method for producing hydrogen. U.S. Patent No: 6,506,360B1
Pera J, Coutaz L, Ambroise J, Chababbet M (1997) Use of incinerator bottom ash in concrete. Cement Concr Res 27:1–5. https://doi.org/10.1016/S0008-8846(96)00193-7
Saffarzadeh A, Arumugam N, Shimaoka T (2016) Aluminum and aluminum alloys in municipal solid waste incineration (MSWI) bottom ash: a potential source for the production of hydrogen gas. Int J Hydrogen Energy 41:820–831. https://doi.org/10.1016/J.IJHYDENE.2015.11.059
Lowson R (1974) Aluminium corrosion studies. I. Potential-pH-temperature diagrams for aluminium. Aust J Chem 27:105. https://doi.org/10.1071/CH9740105
Saikia N, Mertens G, Van Balen K, Elsen J, Van Gerven T, Vandecasteele C (2015) Pre-treatment of municipal solid waste incineration (MSWI) bottom ash for utilisation in cement mortar. Constr Build Mater 96:76–85. https://doi.org/10.1016/J.CONBUILDMAT.2015.07.185
Chaklader ACD (2003) Hydrogen generation from water split reaction
Deng Z-Y, Liu Y-F, Tanaka Y, Ye J, Sakka Y (2005) Modification of Al particle surfaces by gamma-Al2O3 and its effect on the corrosion behavior of Al. J Am Ceram Soc 88:977–979. https://doi.org/10.1111/j.1551-2916.2005.00154.x
Müller U, Rübner K (2006) The microstructure of concrete made with municipal waste incinerator bottom ash as an aggregate component. Cement Concr Res 36:1434–1443. https://doi.org/10.1016/J.CEMCONRES.2006.03.023
Itoh T, Uchida T, Matsubara I, Izu N, Shin W, Miyazaki H et al (2015) Preparation of γ-alumina large grain particles with large specific surface area via polyol synthesis. Ceram Int 41:3631–3638. https://doi.org/10.1016/j.ceramint.2014.11.028
Pan F, Lu X, Wang T, Wang Y, Zhang Z, Yan Y et al (2013) Synthesis of large-mesoporous γ-Al2O3 from coal-series kaolin at room temperature. Mater Lett 91:136–138. https://doi.org/10.1016/J.MATLET.2012.09.052
Pourbaix M (1966) Atlas of electrochemical equilibria in aqueous solutions, 1st edn. Pergamon Press, Oxford
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mathews, G., Moazeni, F. & Smolinski, R. Treatment of reclaimed municipal solid waste incinerator sands using alkaline treatments with mechanical agitation. J Mater Cycles Waste Manag 22, 1630–1638 (2020). https://doi.org/10.1007/s10163-020-01053-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-020-01053-y