Abstract
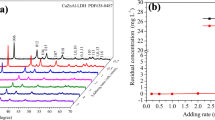
In the present work, the recovery of zinc granules from synthetic electroplating wastewater was evaluated using fluidized-bed homogeneous crystallization. The effect of carbonate-to-zinc ([CO32−]/[Zn2+]) molar ratio (1.0–2.0), precipitant pH (10.30–11.20), initial zinc concentration (100–500 mg/L), and anions (Cl−, F−, and NO3−) on the removal and granulation efficiencies of zinc was investigated. Results show that the highest granulation efficiency of 96.70% was attained at an influent zinc concentration of 300 mg/L, precipitant pH of 10.60 and [CO32−]/[Zn2+] of 1.2. Meanwhile, the highest removal efficiency of 99.90% was obtained at a precipitant pH of 10.60, [CO32−]/[Zn2+] of 1.2, and influent zinc concentration of 100 mg/L. Moreover, the residual zinc concentration of 0.15 mg/L was attained in the treated effluent, which is within the maximum contaminant level of 5.0 mg/L set by the US Environmental Protection Agency and World Health Organization. The presence of anions had little but insignificant effect on the removal where the treated effluent has a residual zinc concentration of 0.44 mg/L. Based on the X-ray diffraction analysis, zinc granules were recovered in the form of smithsonite and hydrozincite with rhombohedral-hexagonal and monoclinic structures, respectively. A broad size distribution was displayed by zinc granules where majority of the pellet diameters fall within the range of 0.149–2.000 mm. Overall, fluidized-bed homogeneous crystallization produced higher-purity pellets and proved to be an effective alternative to seeded crystallization technology.








Similar content being viewed by others
References
Andalib M, Elbeshbishy E, Mustafa N, Hafez H, Nakhla G, Zhu J (2014) Performance of an anaerobic fluidized bed bioreactor (AnFBR) for digestion of primary municipal wastewater treatment biosolids and bioethanol thin stillage. Renew Energy 71:276–285
Ballesteros FC, Salcedo AFS, Vilando AC, Huang YH, Lu MC (2016) Removal of nickel by homogeneous granulation in a fluidized-bed reactor. Chemosphere 164:59–67
Boryczko B, Hołda A, Kolenda Z (2014) Depletion of the non-renewable natural resource reserves in copper, zinc, lead and aluminium production. J Clean Prod 84:313–321
Brackin MJ, Mckenzie DE, Hughes BM, Heitkamp MA (1996) Laboratory-scale evaluation of fluidized bed reactor technology for biotreatment of maleic anhydride process wastewater. J Ind Microbiol 16:216–223
Chen CS, Shih YJ, Huang YH (2015) Remediation of lead (Pb(II)) wastewater through recovery of lead carbonate in a fluidized-bed homogenous crystallization (FBHC) system. Chem Eng J 279:120–128
Chen X, Ren P, Li T, Trembly JP, Liu X (2018) Zinc removal from model wastewater by electrocoagulation: processing, kinetics and mechanism. Chem Eng J 349:358–367
Chicagov AV, Belonozhko AB, Lopatin AL, Dokina TN, Samokhvalova OL, Ushakovskaya TV, Shilova ZV (1990) Information-calculating system on crystal structure data for minerals (MINICRYST). Kristallografiya 35:610–616
Chung J, Jeong E, Choi JW, Yun ST, Maeng SK, Hong SW (2015) Factors affecting crystallization on copper sulfide in fed-batch fluidized bed reactor. Hydrometallurgy 152:107–112
Downs B, Swaminathan R, Bartelmehs K (1993) Crystallographic tables for the rhombohedral carbonates. Am Mineral 78:1104–1107
Ghnimi SM, Frini-Srasra N (2018) A comparison of single and mixed pillared clays for zinc and chromium cations removal. Appl Clay Sci 158:150–157
Ghose S (1964) The crystal structure of hydrozincite, Zn5(OH)6(CO3)2. Acta Cryst 17:1051–1057
Guevara HPR, Ballesteros FC, Vilando AC, de Luna MDG, Lu MC (2017) Recovery of oxalate from bauxite wastewater using fluidized-bed homogeneous granulation process. J Clean Prod 154:130–138
Guillard D, Lewis AE (2001) Nickel carbonate precipitation in a fluidized-bed reactor. Ind Eng Chem Res 40:5564–5569
Hambidge KM, Krebs NF (2007) Zinc deficiency: a special challenge. J Nutr 137:1101–1105
Hosseini SS, Bringas E, Tan NR, Ortiz I, Ghahramani M, Shahmirzadi MAA (2016) Recent progress in the development of high performance polymeric membranes and materials for metal plating wastewater treatment: a review. J Water Process Eng 9:78–110
Kobya M, Demirbas E, Ozyonar F, Sirtbas G, Gengec E (2017) Treatments of alkaline non-cyanide, alkaline cyanide and acidic zinc electroplating wastewaters by electrocoagulation. Process Saf Environ Prot 105:373–385
Kunicky Z, Jandova J, Dostal J, Dvorak J (2008) Zinc recovery from wastes using spent acid from scrapped lead acid batteries. The Southern African Institute of Mining and Metallurgy. http://www.saimm.co.za/Conferences/PbZn2008/000-Prelims.pdf. Accessed 11 June 2019
Lee CI, Yang WF (2005) Heavy metal removal from aqueous solution in sequential fluidized-bed reactors. Environ Technol 26:1345–1354
Lee C, Yang W, Hsieh C (2004) Removal of Cu (II) from aqueous solution in a fluidized-bed reactor. Chemosphere 57:1173–1180
Martín-Lara MA, Blázquez G, Trujillo MC, Pérez A, Calero M (2014) New treatment of real electroplating wastewater containing heavy metal ions by adsorption onto olive stone. J Clean Prod 81:120–129
Mokone TP, van Hille RP, Lewis AE (2012) Metal sulphides from wastewater: assessing the impact of supersaturation control strategies. Water Res 46:2088–2100
Moussavi G, Talebi S (2012) Comparing the efficacy of a novel waste-based adsorbent with PAC for the simultaneous removal of Cr(VI) and cyanide from electroplating wastewater. Chem Eng Res Des 90:960–966
Naito W, Kamo M, Tsushima K, Iwasaki Y (2010) Exposure and risk assessment of zinc in Japanese surface waters. Sci Total Environ 408:4271–4284
Nriagu J (2011) Encyclopedia of environmental health: zinc toxicity in humans. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-444-52272-6.00675-9
Park Y, Kim SH, Matalon S, Wang NHL, Franses EI (2009) Effect of phosphate salts concentrations, supporting electrolytes, and calcium phosphate salt precipitation on the pH of phosphate buffer solutions. Fluid Phase Equilib 278:76–84
Patterson JW, Allen HE, Scala JJ (1977) Carbonate precipitation for heavy metals pollutants. J Water Pollut Control Fed 49:2397–2410
Peng ZX, He HJ, Yang CP, Zeng GM, Wen S, Yan Z, Xiang HH, Cheng Y, Tarre S, Green M (2017) Biological treatment of wastewater with high concentrations of zinc and sulfate ions from zinc pyrithione synthesis. Trans Nonferrous Met Soc China 27:2481–2491
Pereira FV, Gurgel LVA, Gil LF (2010) Removal of Zn2+ from aqueous single metal solutions and electroplating wastewater with wood sawdust and sugarcane bagasse modified with EDTA dianhydride (EDTAD). J Hazard Mater 176:856–863
Plum LM, Rink L, Hajo H (2010) The essential toxin: impact of zinc on human health. Int J Environ Res Public Health 7:1342–1365
Rubio J, Tessele F (1997) Removal of heavy metal ions by adsorptive particulate flotation. Min Eng 10:671–679
Sanna R, Medas D, Podda F, Meneghini C, Casu M, Lattanzi P, Scoriapino MA, Floris C, Cannas C, de Giudici G (2015) Binding of bis-(2-ethylhexyl)phthalate at the surface of hydrozincite nanocrystals: an example of organic molecules absorption onto nanocrystalline minerals. J Colloid Interface Sci 457:298–306
Senthilkumar R, Vijayaraghavan K, Thilakavathi M, Iyer PVR, Velan M (2006) Seaweeds for the remediation of wastewaters contaminated with zinc(II) ions. J Hazard Mater 136:791–799
Shimizu Y, Hirasawa I (2013) Impurities effect on carbonate reactive crystallization for the wastewater. ISRN Chem Eng 1–8
Sönmez S, Aktas S, Açma E (2003) A study on the treatment of wastes in hot dip galvanizing plants. Can Metall Q 42:289–300
Stefanidou M, Maravelias C, Dona A, Spiliopoulou C (2008) Zinc: a multipurpose trace element. Cancer Sci 99:1515–1522
Stoilova D, Koleva V, Vasileva V (2002) Infrared study of some synthetic phases of malachite (Cu2(OH)2CO3)-hydrozincite (Zn5(OH)6(CO3)2) series. Spectrochim Acta, Part A 58:2051–2059
Tai CY, Chien WC, Chen CY (1999) Crystal growth kinetics of calcite in a dense fluidized-bed crystallizer. Am Inst Chem Eng J 45:1605–1614
Tai CY, Chen PC, Tsao TM (2006) Growth kinetics of CaF2 in a pH-stat fluidized-bed crystallizer. J Cryst Growth 290:576–584
US Environmental Protection Agency (2014) Guidelines for Water Reuse. US Agency for International Development, Washington
Van Dijk JC, Van Ammers M, Graveland A, Nuhn PANM (1986) State of the art of pellet softening in The Netherlands. Water Supply 4:223–235
Vilando AC, Caparanga AR, Huang YH, Lu MC (2017) Tohdite recovery from water by fluidized-bed homogeneous granulation process. Desalin Water Treat 96:224–230
World Health Organization (2011) Guidelines for drinking-water quality, 4th edn. WHO Press, Geneva, pp 433–434
Ye X, Ye ZL, Lou Y, Pan S, Wang X, Wang MK, Chen S (2016) A comprehensive understanding of saturation index and upflow velocity in a pilot-scale fluidized bed reactor for struvite recovery from swine wastewater. Powder Technol 295:16–26
Zhang J, Xu Y, Zhou J, Liang Y, Chen C, Liu Q, Qian G, Xu ZP (2013) Magnetic nanomaterials recovered from co-treatment of CN-containing electroplating wastewaters and pickle acid liquor. Sep Purif Technol 120:186–190
Zumdahl SS (2009) Chemical Principles, 6th edn. Massachusetts, Boston
Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan, under Grant MOST 102-2221-E-041-001-MY3 and National Research Foundation (NRF) of Korea through Ministry of Education under Grant 2016R1A6A1A03012812.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: J Aravind.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Luna, M.D.G., Paulino, L.H.S., Futalan, C.M. et al. Recovery of zinc granules from synthetic electroplating wastewater using fluidized-bed homogeneous crystallization process. Int. J. Environ. Sci. Technol. 17, 129–142 (2020). https://doi.org/10.1007/s13762-019-02439-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02439-8