Abstract
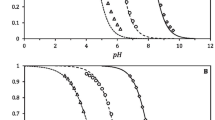
The Hill plots of NMR titration data for protein residues disclose more clearly than the usual titration curves the presence of multiple weak perturbations originating from other titratable groups, and should be used whenever the conventional curve fitting is poor. For a quantitative interpretation, we derive here expressions for the Hill equation and the Hill coefficient when the titration of the observed group is perturbed by more than one titratable group. When the generalized Hill equation is fitted to the data, values of the interaction parameters between the observed group and the others are extracted provided that there are no mutual interactions between the latter groups. The method is applied to the titration data of two histidyl residues of l-arginine phosphotransferase (E.C. 2.7.3.3.) in the transition state analogue complex (enzyme-Mg2+-ADP-NOsk3/−l-Arg). From the Hill plots, interactions with three titratable groups are disclosed for both residues, and the fitting with the Hill equation reveals that they experience perturbations from the same three groups. Microscopic pK values are obtained for all the involved groups, indicating large changes (up to 3 pH units) upon protonation of the interacting groups. As compared to the conventional fitting procedure, the use and fitting of Hill plots yields from NMR data more information on the neighbourhood of enzyme residues and on the changes intervening therein through the steps involved in the catalysis.
Similar content being viewed by others
References
Bovey FA (1969) NMR spectroscopy. Academic Press, New York, p 64
Dahlquist FW (1974) The quantitative interpretation of maximum in Scatchard plots. FEBS Lett 49:267–268
Emsley JW, Feeney J, Sutcliffe LH (1965) High resolution NMR, vol 1. Pergamon Press, Oxford, p 485
Hill AV (1910) The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol 40:IV-VIII
Markley JL (1975) Observation of histidine residues in proteins by means of NMR spectroscopy. Acc Chem Res 8:70–80
Milner-White EJ, Watts DC (1971) Inhibition of adenosine 5′-triphosphate-creatine phosphotransferase by substrate-anion complexes. Evidence for the transition state organization of the catalytic site. Biochem J 122:727–740
Roustan C, Pradel LA, Kassab R, Fattoum A, Thoai NV (1970) Spectrophotometric investigations of the interaction of native and chemically modified ATP: guanidinophosphotransferases with their substrates. Biochim Biophys Acta 206:369–379
Sachs DH, Schechter AN, Cohen JS (1971) Nuclear magnetic resonance titration curves of histidine ring protons. I. Influence of neighbouring charged groups. J Biol Chem 246:6576–6580
Schwarz G (1976) Some general aspects regarding the interpretation of binding data by means of a Scatchard plot. Biophys Struct Mech 2:1–12
Shrager RI, Cohen JS, Heller SR, Sachs DH, Schechter AN (1972) Mathematical models for interacting groups in NMR titration curves. Biochemistry 11:541–547
Tipton KF, Dixon HBF (1979) Effects of pH on enzymes. In:Purich DL (ed) Methods in enzymology, vol 63. Academic Press, New York, pp 183–213
Wyman J Jr (1948) Linked functions in heme proteins. Adv Protein Chem 4:436–443
Wyman J Jr (1967) Allosteric linkage. J Am Chem Soc 89:2202–2218
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Roux-Fromy, M. On the Hill plot of NMR data for titration of protein residues. Biophys. Struct. Mechanism 8, 289–306 (1982). https://doi.org/10.1007/BF00537207
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00537207