Abstract
Aim and background
Postoperative nausea and vomiting (PONV) remains a significant clinical problem for surgical patients. Amisulpride is a well-studied D2/D3 antagonist that has the potential to be used for preventing and treating PONV. Our aim was to assess the efficacy and safety of amisulpride for prevention and treatment of PONV through a systematic review and meta-analysis.
Method
A systematic literature search was performed using MEDLINE, EMBASE, PUBMED, clinicaltrials.gov, and the Cochrane Central Register of Controlled Trials from their inception to Feb 15th, 2019. The efficacy outcome was the incidence of complete response, defined as no emesis and no rescue antiemetic use in a 24-h period after study drug administration. The safety outcomes were the adverse effects associated with amisulpride.
Results
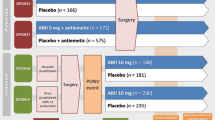
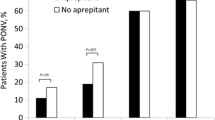
Five studies comprising 3243 patients met inclusion critieria. Compared with placebo, amisulpride showed a significantly improved incidence of complete response [relative risk (RR): 1.30; 95% confidence interval (CI): 1.20–1.41; P < 0.00001, I2 = 0%] with firm evidence from the trial sequential analysis. Particularly, the amisulpride at 5 mg dose indicated a significant benefit than placebo [relative risk (RR): 1.28; 95% confidence interval (CI): 1.18–1.39; P < 0.00001, I2 = 4%]. The adverse event profile of amisulpride was generally similar to the placebo.
Conclusion
Based on our findings, low-dose, intravenous amisulpride is safe and efficacious for the prevention and treatment of PONV compared to placebo. Further studies are needed to explore the optimal dose and timing.
Clinical trial registration
PROSPERO: CRD42019121483.




Similar content being viewed by others
References
Gan TJ (2002) Postoperative nausea and vomiting—-can it be eliminated? JAMA 287(10):1233–1236. https://doi.org/10.1001/jama.287.10.1233
Herrstedt J, Summers Y, Daugaard G, Christensen TB, Holmskov K, Taylor PD, Fox GM, Molassiotis A (2018) Amisulpride in the prevention of nausea and vomiting induced by cisplatin-based chemotherapy: a dose-escalation study. Support Care Cancer 26(1):139–145. https://doi.org/10.1007/s00520-017-3825-2
Apfel CC, Korttila K, Abdalla M, Biedler A, Kranke P, Pocock SJ, Roewer N (2003) An international multicenter protocol to assess the single and combined benefits of antiemetic interventions in a controlled clinical trial of a 2x2x2x2x2x2 factorial design (IMPACT). Control Clin Trials 24(6):736–751. https://doi.org/10.1016/s0197-2456(03)00107-7
Habib AS, Gan TJ (2008) The use of droperidol before and after the Food and Drug Administration black box warning: a survey of the members of the Society of Ambulatory Anesthesia. J Clin Anesth 20(1):35–39. https://doi.org/10.1016/j.jclinane.2007.08.003
Gan TJ, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, Hooper VD, Kovac AL, Kranke P, Myles P, Philip BK, Samsa G, Sessler DI, Temo J, Tramer MR, Vander Kolk C, Watcha M (2007) Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg 105(6):1615–1628, table of contents. https://doi.org/10.1213/01.ane.0000295230.55439.f4
Kranke P, Eberhart L, Motsch J, Chassard D, Wallenborn J, Diemunsch P, Liu N, Keh D, Bouaziz H, Bergis M, Fox G, Gan TJ (2013) I.V. APD421 (amisulpride) prevents postoperative nausea and vomiting: a randomized, double-blind, placebo-controlled, multicentre trial. Br J Anaesth 111(6):938–945. https://doi.org/10.1093/bja/aet251
Juruena MF, de Sena EP, de Oliveira IR (2010) Safety and tolerability of antipsychotics: focus on amisulpride. Drug Healthc Patient Saf 2:205–211. https://doi.org/10.2147/dhps.S6226
Rein W, Coulouvrat C, Dondey-Nouvel L (2000) Safety profile of amisulpride in short- and long-term use. Acta Psychiatr Scand Suppl 400:23–27. https://doi.org/10.1111/j.0065-1591.2000.007s021[dash]5.x
Taubel J, Ferber G, Fox G, Fernandes S, Lorch U, Camm AJ (2017) Thorough QT study of the effect of intravenous amisulpride on QTc interval in Caucasian and Japanese healthy subjects. Br J Clin Pharmacol 83(2):339–348. https://doi.org/10.1111/bcp.13128
Joy JP, Coulter CV, Duffull SB, Isbister GK (2011) Prediction of torsade de pointes from the QT interval: analysis of a case series of amisulpride overdoses. Clin Pharmacol Ther 90(2):243–245. https://doi.org/10.1038/clpt.2011.107
Habib AS, Kranke P, Bergese SD, Chung F, Ayad S, Siddiqui N, Motsch J, Leiman DG, Melson TI, Diemunsch P, Fox GM, Candiotti KA (2019) Amisulpride for the rescue treatment of postoperative nausea or vomiting in patients failing prophylaxis: a randomized, placebo-controlled phase III trial. Anesthesiology 130(2):203–212. https://doi.org/10.1097/aln.0000000000002509
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135. https://doi.org/10.1186/1471-2288-14-135
Takwoingi Y, Hopewell S, Tovey D, Sutton AJ (2013) A multicomponent decision tool for prioritising the updating of systematic reviews. BMJ 347:f7191. https://doi.org/10.1136/bmj.f7191
Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64(4):401–406. https://doi.org/10.1016/j.jclinepi.2010.07.015
Kranke P, Bergese SD, Minkowitz HS, Melson TI, Leiman DG, Candiotti KA, Liu N, Eberhart L, Habib AS, Wallenborn J, Kovac AL, Diemunsch P, Fox G, Gan TJ (2018) Amisulpride prevents postoperative nausea and vomiting in patients at high risk: a randomized, double-blind, placebo-controlled trial. Anesthesiology 128(6):1099–1106. https://doi.org/10.1097/aln.0000000000002133
Kranke P, Eberhart L, Motsch J, Chassard D, Wallenborn J, Diemunsch P, Liu N, Keh D, Bouaziz H, Bergis M, Fox G, Gan TJ (2013) I.V. APD421 (amisulpride) prevents postoperative nausea and vomiting: a randomized, double-blind, placebo-controlled, multicentre trial. Br J Anaesth 111(6):938–945. https://doi.org/10.1093/bja/aet251
Candiotti KA, Kranke P, Bergese SD, Melson TI, Motsch J, Siddiqui N, Chung F, Rodriguez Y, Minkowitz HS, Ayad SS, Diemunsch P, Fox G (2019) Randomized, double-blind, placebo-controlled study of intravenous amisulpride as treatment of established postoperative nausea and vomiting in patients who have had no prior prophylaxis. Anesth Analg 128(6):1098–1105. https://doi.org/10.1213/ane.0000000000003733
Gan TJ, Kranke P, Minkowitz HS, Bergese SD, Motsch J, Eberhart L, Leiman DG, Melson TI, Chassard D, Kovac AL, Candiotti KA, Fox G, Diemunsch P (2017) Intravenous amisulpride for the prevention of postoperative nausea and vomiting: two concurrent, randomized, double-blind, placebo-controlled trials. Anesthesiology 126(2):268–275. https://doi.org/10.1097/aln.0000000000001458
Kovac AL (2018) Updates in the management of postoperative nausea and vomiting. Adv Anesth 36(1):81–97. https://doi.org/10.1016/j.aan.2018.07.004
Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, Watcha M, Chung F, Angus S, Apfel CC, Bergese SD, Candiotti KA, Chan MT, Davis PJ, Hooper VD, Lagoo-Deenadayalan S, Myles P, Nezat G, Philip BK, Tramer MR (2014) Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 118(1):85–113. https://doi.org/10.1213/ane.0000000000000002
Koivuranta M, Laara E (1998) A survey of postoperative nausea and vomiting. Anaesthesia 53(4):413–414
Herrstedt J, Summers Y, Jordan K, von Pawel J, Jakobsen AH, Ewertz M, Chan S, Naik JD, Karthaus M, Dubey S, Davis R, Fox GM (2018) Amisulpride prevents nausea and vomiting associated with highly emetogenic chemotherapy: a randomised, double-blind, placebo-controlled, dose-ranging trial. Support Care Cancer 27(7):2699–2705 https://doi.org/10.1007/s00520-018-4564-8
Habib AS, Gan TJ (2005) The effectiveness of rescue antiemetics after failure of prophylaxis with ondansetron or droperidol: a preliminary report. J Clin Anesth 17(1):62–65. https://doi.org/10.1016/j.jclinane.2004.04.004
Habib AS, Reuveni J, Taguchi A, White WD, Gan TJ (2007) A comparison of ondansetron with promethazine for treating postoperative nausea and vomiting in patients who received prophylaxis with ondansetron: a retrospective database analysis. Anesth Analg 104(3):548–551. https://doi.org/10.1213/01.ane.0000252433.73485.be
Habib AS, Chen YT, Taguchi A, Hu XH, Gan TJ (2006) Postoperative nausea and vomiting following inpatient surgeries in a teaching hospital: a retrospective database analysis. Curr Med Res Opin 22(6):1093–1099. https://doi.org/10.1185/030079906x104830
Candiotti KA, Ahmed SR, Cox D, Gan TJ (2014) Palonosetron versus ondansetron as rescue medication for postoperative nausea and vomiting: a randomized, multicenter, open-label study. BMC Pharmacol Toxicol 15:45. https://doi.org/10.1186/2050-6511-15-45
Fox GM, Albayaty M, Walker JL, Xue H, Darpo B (2019) Intravenous amisulpride does not meaningfully prolong the QTc interval at doses effective for the management of postoperative nausea and vomiting. Anesth Analg. https://doi.org/10.1213/ane.0000000000004538
Canal M, Fraisse J, Thenot JP (2002) Amisulpride. Metabolic and pharmacokinetic profile after 14C oral administration 12(02):319–319. https://doi.org/10.1016/S0924-977X(02)80480-1
Gillet G, Dormerque L, Canal M, Thénot JP (2000) Amisulpride does not inhibit cytochrome P450 isozymes. Eur Neuropsychopharmacol 10:331–332. https://doi.org/10.1016/S0924-977X(00)80407-1
Chrisp P, Goa KL (1990) Nafarelin. A review of its pharmacodynamic and pharmacokinetic properties, and clinical potential in sex hormone-related conditions. Drugs 39(4):523–551. https://doi.org/10.2165/00003495-199039040-00005
Spina E, de Leon J (2007) Metabolic drug interactions with newer antipsychotics: a comparative review. Basic Clin Pharmacol 100(1):4–22. https://doi.org/10.1111/j.1742-7843.2007.00017.x
Chiu L, Chow R, Popovic M, Navari RM, Shumway NM, Chiu N, Lam H, Milakovic M, Pasetka M, Vuong S, Chow E, DeAngelis C (2016) Efficacy of olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis. Support Care Cancer 24(5):2381–2392. https://doi.org/10.1007/s00520-016-3075-8
Yoodee J, Permsuwan U, Nimworapan M (2017) Efficacy and safety of olanzapine for the prevention of chemotherapy-induced nausea and vomiting: a systematic review and meta-analysis. Crit Rev Oncol Hematol 112:113–125. https://doi.org/10.1016/j.critrevonc.2017.02.017
Canal M, Chaufour S, Lavanant C, Zieleniuk I, Piette JF, Deschamps C (2000) Amisulpride pharmacokinetics: no difference between young and elderly subjects. Eur Neuropsychopharmacol 10(Suppl 3):331. https://doi.org/10.1016/S0924-977X(00)80406-X
Canal M, Macmahon M, Kwan J, Dubruc C (2000) Amisulpride: kinetics in patients with renal failure. Eur Neuropsychopharmacol 10(Suppl 3):330. https://doi.org/10.1016/S0924-977X(00)80404-6
Author information
Authors and Affiliations
Contributions
Name: Lu-Feng Zhang, M.Med.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Name: Chao-Fan Zhang, M.Med.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Name: Wen-Xin Tang, MD.
Contribution: This author helped analyze the data.
Name: Long He, MD.
Contribution: This author helped analyze the data and write the manuscript.
Name: Yang Liu, M.Med.
Contribution: This author helped analyze the data.
Name: Dan-dan Tian, MD.
Contribution: This author helped design the study and conduct the study.
Name: Yan-Qiu Ai, MD.
Contribution: This author helped design the study, conduct the study, and write the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, LF., Zhang, CF., Tang, WX. et al. Efficacy of amisulpride on postoperative nausea and vomiting: a systematic review and meta-analysis. Eur J Clin Pharmacol 76, 903–912 (2020). https://doi.org/10.1007/s00228-020-02869-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02869-1