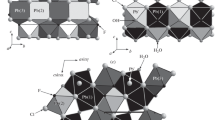
Abstract
The crystal structure of a new mineral britvinite Pb7.1Mg4.5(Si4.8Al0.2O14)(BO3)(CO3)[(BO3)0.7(SiO4)0.3](OH, F)6.7 from the Lángban iron-manganese skarn deposit (Värmland, Sweden) is determined at T = 173 K using X-ray diffraction (Stoe IPDS diffractometer, λMoKα, graphite monochromator, 2θmax = 58.43°, R = 0.052 for 6262 reflections). The main crystal data are as follows: a = 9.3409(8) Å, b = 9.3579(7) Å, c = 18.8333(14) Å, α = 80.365(6)°, β = 75.816(6)°, γ = 59.870(5)°, V = 1378.7(2) Å3, space group P1, Z = 2, and ρcalcd = 5.42 g/cm3. The idealized structural formula of the mineral is represented as [Pb7(OH)3F(BO3)2(CO3)][Mg4.5(OH)3(Si5O14)]. It is demonstrated that the mineral britvinite is a new representative of the group of mica-like layered silicates with structures in which three-layer (2: 1) “sandwiches” composed of tetrahedra and octahedra alternate with blocks of other compositions, such as oxide, oxide-carbonate, oxide-carbonate-sulfate, and other blocks. The tetrahedral networks (Si5O14)∞∞ consisting of twelve-membered rings are fragments of the britvinite structure. Similar networks also form crystal structures of the mineral zeophyllite and the synthetic phase Rb6Si10O23. In the crystal structures under consideration, the tetrahedral networks differ in the rotation of tetrahedra with respect to the layer plane.
Similar content being viewed by others
References
N. V. Chukanov, O. V. Yakubovich, I. V. Pekov, et al., Zap. Vseross. Mineral. O-va, Part 136, No. 6, 18 (2007).
STOE and Cie: Imaging Plate Diffraction System (IPDS), Version 2.90 (STOE and Cie, Darmstadt, Germany, 1999).
G. M. Sheldrick, SHELXS97: Program for the Solution of Crystal Structures (University of Göttingen, Göttingen, Germany, 1997).
G. M. Sheldrick, SHELXL97: Program for the Refinement of Crystal Structures from Diffraction Data (University of Göttingen, Göttingen, Germany, 1997).
International Tables for Crystallography, Vol. C: Mathematical, Physical, and Chemical Tables, Ed. by E. Prince, 3rd ed. (Kluwer, Dordrecht, The Netherlands, 2004), Tables 4.2.6.8 and 6.1.14.
O. V. Yakubovich, I. M. Still, P. G. Gavrilenko, and V. S. Urusov, Kristallografiya 50(2), 226 (2005) [Crystallogr. Rep. 50 (2), 194 (2005)].
O. Crottaz, F. Kubel, and H. Schmid, J. Solid State Chem. 120, 60 (1995).
S. V. Krivovichev and C. Burns, Mineral. Mag. 64, 1069 (2000).
G. Giuseppetti, F. Mazzi, and C. Tadini, Neues Jahrb. Mineral., Monatsh., No. 6, 255 (1990).
I. M. Steele, J. J. Pluth, and A. Livingstone, Mineral. Mag. 62, 451 (1998).
I. M. Steele, J. J. Pluth, and A. Livingstone, Eur. J. Mineral. 11, 493 (1999).
H. I. Strunz and E. N. Nickel, Strunz Mineralogical Tables (E. Schweizerbart’sche, Stuttgart, Germany, 2001).
S. P. Saburov, S. N. Britvin, G. K. Bekenova, et al., Am. Mineral. 90, 1163 (2005).
M. Uehara, A. Yamazaki, and S. Tsutsumi, Am. Mineral. 82, 416 (1997).
A. R. Kampf, L. L. Jackson, G. B. Sidder, et al., Am. Mineral. 77, 1107 (1992).
P. J. Dunn, R. S. W. Braithwaite, A. C. Roberts, and R. A. Ramik, Am. Mineral. 75, 702 (1990).
M. J. Wilson, J. D. Russel, J. M. Tait, et al., Mineral. Mag. 48, 127 (1984).
R. V. Gaines, P. B. Leavens, and J. A. Nelen, Am. Mineral. 64, 355 (1979).
D. E. Appleman, H. T. Evans, G. L. Nord, et al., Mineral. Mag. 51, 417 (1987).
S. Merlino, Acta Crystallogr., Sect. B: Struct. Sci. 28, 2726 (1972).
W. Mikenda, F. Pertlik, P. Povondra, and J. Ulrych, Mineral. Petrol. 61, 199 (1997).
N. V. Belov, Essays on Structural Mineralogy (Nedra, Moscow, 1976) [in Russian].
A. E. Lapshin, N. V. Borisova, V. M. Ushakov, and Yu. F. Shepelev, Zh. Neorg. Khim. 51(3), 487 (2006) [Russ. J. Inorg. Chem. 51 (3), 438 (2006)].
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.V. Yakubovich, W. Massa, N.V. Chukanov, 2008, published in Kristallografiya, 2008, Vol. 53, No. 2, pp. 233–242.
Rights and permissions
About this article
Cite this article
Yakubovich, O.V., Massa, W. & Chukanov, N.V. Crystal structure of britvinite [Pb7(OH)3F(BO3)2(CO3)][Mg4.5(OH)3(Si5O14)]: A new layered silicate with an original type of silicon-oxygen networks. Crystallogr. Rep. 53, 206–215 (2008). https://doi.org/10.1134/S1063774508020077
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774508020077