Summary
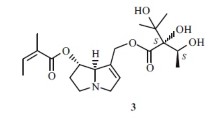
The structure 3-butyl-5-propylindolizidine (2), tentatively assigned to a minor alkaloid in skin extracts from a number of poison frogs of the Neotropical genusDendrobates, has been confirmed and its stereochemistry determined as 5E, 9E (2d) by comparison on GC and GC-MS with the four synthetic diastereomers2a-2d.
Similar content being viewed by others
References
J.W. Daly, G.B. Brown, M. Mensah-Dwumah and C.W. Myers, Toxicon16, 163 (1978). See discussion for GC peak 223AB. The EI spectrum (LKB 3000 spectrometer, 70eV) exhibited a parent ion at m/z 223 (intensity 1% of base peak) and only 2 major fragment ions, one at 180 (loss of C3H7, intensity 66% of base peak) and the other at 166 (loss of C4H9, base peak). This material has been referred to in ref. 14 as GTX-223, since it appeared to be related structurally to gephyrotoxin (GTX)13. Biosynthesis of most dendrobatid alkaloids appears likely to derive from a precursor 2,6-disubstituted piperidine which has odd numbers of carbons in both substituents; cf. histrionicotoxins, pumiliotoxin-C, gephyrotoxin. Because of this, an alternative structure for GTX-223AB, viz. 3-propyl-5-butylindolizidine5, was considered quite unlikely since it would derive from a 2,6-disubstituted piperidine with4 and 6 carbon substituents.
J. W. Daly, T. Tokuyama, R. J. Highet and I. L. Karle, J. Am. chem. Soc.102, 830 (1980).
J.W. Daly, B. Witkop, T. Tokuyama, T. Nishikawa and I.L. Karle, Helv. chim. Acta60, 1128 (1977).
F. J. Ritter, I. E. M. Rotgans, E. Talman, P. E. J. Verwiel and F. Stein, Experientia29, 530 (1973).
J. P. Edwards and D. B. Pinninger, Ann. appl. Biol.89, 395 (1978). We have adopted Sonnet's use13 of the E, Z nomenclature to characterize indolizidine stereoisomers. The configuration of the hydrogen atom at C(5) and C(9) is referred to the hydrogen at C(3) and is either in a cis-(Z) or trans-(E) relationship.
J. E. Oliver and P. E. Sonnet, J. org. Chem.39, 2663 (1974).
P. E. Sonnet and J. E. Oliver, J. het. Chem.12, 289 (1975).
Ref. 2. See e.g. alkaloids 239 AB and 239CD.
Initially diastereomer2d was reported14 as different from GTX-223AB since coinjection with the natural material gave rise to 2 peaks on a 3% OV-17 GC column. We have since been unable to repeat this result and we have no explanation for this discrepancy.
T.F. Spande, unpublished results.
“U”-Shaped 2 mm (i.d.)×150 cm GC columns of 3% OV-17 (100–120 mesh) (Applied Science Laboratories, State College, PA) (column A) or 10% SP-1000 on Supelcoport (100–120 mesh) (Supelco, Inc., Bellefonte, PA) (column B). A Finnegan gas chromatograph, model 9500 (injection port 250°) equipped with a flame ionization detector (300°) was used with a Hewlett-Packard 3380A recorder-integrator. The carrier (N2) flow rate was 18 cc/min. The following are typical retention times for2a, 2b, 2c and2d, respectively on column A (90°C): 16.6, 19.1, 21.2, 22.7 min; column B (95 °C): 17.4, 19.9, 22.7, 24.2 min. Separation of all 4 diastereomers could not be obtained on the following columns: 1.5% OV-1, 3% SE-30, 3% OV-225,3% Poly I-110, and 5% ECNSS. However, in all these cases,2d chromatographed with the natural compound.
D.J. Hart and Y.-M. Tsai, unpublished results. The 5E, 9Z and 5Z, 9E configurations for2b and2c, respectively, could also be assigned on the basis of conformational arguments. Compound2b from the hydrogenation of1 eluted first from silica gel (7% CH3OH−CHCl3).13C NMR (C6D6)2b: 58.4, 56.4, 52.7, 33.5, 33.2, 30.4, 28.8, 28.5 (2), 23.6, 21.3, 20.0, 14.7, 14.4 ppm.2c: 58.5, 54.8, 52.3, 36.3, 35.5, 29.8, 28.9, 28.8, 27.7, 23.6 (2), 20.7, 20.1, 14.6, 14.5 ppm.
2a:13C NMR (CDCl3): 67.7, 65.3, 62.3, 39.6, 38.0, 32.0, 31.0, 30.5, 29.8, 29.2 (2), 25.0, 22.9, 14.4, 14.0 ppm. M.S. (70 eV): m/z 166 (100%), 180 (52%), 222 (3%), 223 (1%). IR (neat): Bohlmann bands (see ref. 13) observed at 2550, 2630, 2700 and 2790 cm−1 in order of increasing intensity. Compound2a eluted last from silica gel (9% CH3OH−CHCl3).
P.E. Sonnet, D.A. Netzel and R. Mendoza, J. het. Chem.16, 1041 (1979).
T.L. Macdonald, J. org. Chem.45, 193 (1980).2d:13C NMR (CDCl3): 58.5, 57.7, 55.9, 34.7, 30.9, 29.6, 28.7, 28.3, 25.4, 24.3, 23.6, 21.9, 17.9, 13.4, 13.0 ppm. The unusual hydrogenation result is discussed above.
GC column A (ref. 10) was used with a Perkin-Elmer Sigma 3 gas chromatograph attached to a V.G. Micromass Ltd., model 7070F mass spectrometer in the EI mode (70 eV). The carrier (He) flow rate was 25 cc/min. Slight differences in m/z 180/160 ratios are evident between EI spectra obtained directly and in the GC-MS mode (cf.2a, ref. 12, fig. 2). We thank Mr. Noel Whittaker (NIAMDD, NIH) for the EI spectra.
The EI spectra for the initial 3 ‘iso’ diastereomers (5a-5c) in order of increasing retention times had the following m/z 166/180 ratios±SEM for (n) scans through the GC peak: 0.42±0.11 (8), 1.98±0.38 (11) and 1.37±0.36 (4). The G.C. peak corresponding to5d had 1.42±0.15 (6) and 1.36±0.37 (7) (2 runs). This corresponds to m/z 180/160 ratios of 0.30±0.10 (12), 1.42±0.35 (14), 1.04±0.19 (6) and 0.89±0.27 (11), for2a,2b,2c and2d, respectively. In both series, the ratios compared are for loss of the 5-substituent/loss of the 3-substituent; i.e., m/z 166/180 for the ‘iso’ compounds and m/z 180/166 for compounds2a-d. These data along with the relative peak areas support the same order of retention times for the ‘iso’ series as observed for2a–2d. The natural material had a 180/160 ratio of 0.93±0.23 (8). The magnitude of these ratios depends very much on the source design of the spectrometer (cf. footnote 2, 15) but it is relatively invariant with the same instrument.
R.J. Abraham and P. Loftus, Proton and Carbon-13 NMR Spectroscopy, p. 25. Heyden, London 1979.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spande, T.F., Daly, J.W., Hart, D.J. et al. The structure of gephyrotoxin (GTX) 223AB. Experientia 37, 1242–1245 (1981). https://doi.org/10.1007/BF01948336
Issue Date:
DOI: https://doi.org/10.1007/BF01948336