Abstract
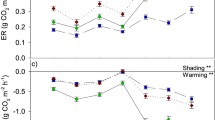
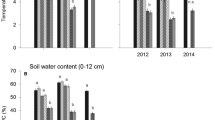
Frost-patterned grounds, such as mostly barren frost boils surrounded by denser vegetation, are typical habitat mosaics in tundra. Plant and microbial processes in these habitats may be susceptible to short-term warming outside the growing season, while the areal cover of barren frost boils has decreased during the past decades due to climate warming-induced shrub expansion. The relative importance of such short-term and long-term climate impacts on carbon (C) dynamics remains unknown. We measured ecosystem CO2 uptake and release (in the field), microbial respiration (in the laboratory), as well as microbial biomass N and soil extractable N in frost boils and the directly adjacent heath in late spring and late summer. These habitats had been experimentally warmed with insulating fleeces from late September until late May for three consecutive years, which allowed us to investigate the direct short-term effects of warming and longer-term, indirect climate effects via vegetation establishment into frost boils. Non-growing season warming increased C uptake at the frost boils in late spring and decreased it in late summer, while the timing and direction of responses was opposite for the heath. Experimental warming had no effects on microbial or ecosystem C release or soil N at either of the habitats. However, C cycling was manifold higher at the heath compared to the frost boils, likely because of a higher SOM stock in the soil. Short-term climate change can thus directly alter ecosystem C uptake at frost-patterned grounds but will most likely not affect microbial C release. We conclude that the C dynamics at frost-patterned grounds under a changing climate depend most strongly on the potential of vegetation to encroach into frost boils in the long-term.




Similar content being viewed by others
References
ACIA (2005) Impacts of a warming Arctic. Arctic climate impact assessment. Cambridge University Press, Cambridge, New York, NY
Becher M, Olid C, Klaminder J (2013) Buried soil organic inclusions in non-sorted circles fields in northern Sweden: age and Paleoclimatic context. J Geophys Res 118:104–111
Becher M, Olofsson J, Klaminder J (2015) Cryogenic disturbance and its impacts on carbon fluxes in a subarctic heathland. Environ Res Lett 10:114006
Blok D, Weijers S, Welker JM, Cooper E, Michelsen A, Löffler J, Elberling B (2015) Deepened winter snow increases stem growth and alters stem δ13C and δ15N in evergreen dwarf shrub Cassiope tetragona in high-arctic Svalbard. Environ Res Lett 10:044008
Bokhorst S, Bjerke JW, Tømmervik H, Preece C, Phoenix GK (2012) Ecosystem response to climatic changes: the importance of the cold season. Ambio 41:246–255
Bokhorst S, Metcalfe DB, Wardle DA (2013) Reduction in snow depth negatively affects decomposers but impact on decomposition rates is substrate dependent. Soil Biol Biochem 62:157–164
Brookes PC, Kragt JF, Powlson DS, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: the effects on fumigation time and temperature. Soil Biol Biochem 17:831–835
Brostoff WN, Sharifi MR, Rundel PW (2005) Photosynthesis of cryptobiotic soil crusts in a seasonally inundated system of pans and dunes in the western Mojave Desert, CA: field studies. Flora 200:592–600
Cooper EJ (2014) Warmer shorter winters disrupt arctic terrestrial ecosystems. Annu Rev Ecol Evol Syst 45:271–295
Cornelissen JHC, Callaghan TV, Alatalo JM et al (2001) Global change and arctic ecosystems: is lichen decline a function of increases in vascular plant biomass? J Ecol 89:984–994
Creamer RE, Schulte RPO, Stone D et al (2014) Measuring basal soil respiration across Europe: do incubation temperature and incubation period matter? Ecol Indic 36:409–418
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
DeMarco J, Mack MC, Bret-Harte MS (2014) Effects of arctic shrub expansion on biophysical vs. biogeochemical drivers of litter decomposition. Ecology 95:1861–1875
Dickson LG (2000) Constraints to nitrogen fixation by cryptogamic crusts in polar desert ecosystem, Devon Island, NWT, Canada. Arct Antarct Alp Res 32:40–45
Elmendorf SC, Henry GHR, Hollister RD, Björk RG (2012a) Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat Clim Change 2:453–457
Elmendorf SC, Henry GHR, Hollister RD et al (2012b) Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol Lett 15:164–175
Emmerton CA, St. Louis VL, Humphreys ER, Gamon JA, Barker JD, Pastorellos GZ (2016) Net ecosystem exchange of CO2 with rapidly changing high Arctic landscapes. Glob Change Biol 22:1185–1200
Evans RD, Lange OL (2001) Biological soil crusts and ecosystem nitrogen and carbon dynamics. In: Balnap J, Lange OL (eds) Biological soil crusts: structure, function and management. Springer, Berlin Heidelberg, pp 263–279
Finzi AC, Abramoff RZ, Spiller KS, Brzostek ER, Darby BA, Kramer MA, Phillips RP (2015) Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob Change Biol 21:2081–2094
Frost GV, Epstein HE (2014) Tall shrub and tree expansion in Siberian tundra ecotones since the 1960s. Glob Change Biol 20:1264–1277
Gold WG, Glew KA, Dickson LG (2001) Functional influences of cryptobiotic surface crusts in an alpine tundra basin of the Olympic Mountains, Washington, U.S.A. Northwest Sci 75:315–326
Hjort J, Luoto M (2009) Interaction of geomorphic and ecologic features across altitudinal zones in a subarctic landscape. Geomorphology 112:324–333
Hugelius G, Strauss J, Zubrzycki S et al (2014) Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences 11:6573–6593
Huo C, Luo Y, Cheng W (2017) Rhizosphere priming effect: a meta-analyses. Soil Biol Biochem 111:78–84
Inouye DW (2008) Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89:353–362
Iversen CM, Sloan VL, Sullivan PF et al (2015) The unseen iceberg: plant roots in arctic tundra. New Phytol 205:34–58
Johansson M, Christensen TR, Akerman HJ, Callaghan TV (2006) What determines the current presence or absence of permafrost in the Torneträsk region, a sub-arctic landscape in Northern Sweden? Ambio 35:190–197
Kade A, Walker DA, Raynolds MK (2005) Plant communities and soils in cryoturbated tundra along a bioclimate gradient in the Low Arctic, Alaska. Phytocoenologia 35:761–820
Klaminder J, Giesler R, Makoto K (2013) Physical mixing between humus and mineral matter found in cryoturbated soils increases short-term heterotrophic respiration. Soil Biol Biochem 57:922–924
Krab EJ, Rönnefarth J, Becher M, Blume-Werry G, Keuper F, Klaminder J, Kreyling J, Makoto K, Milbau A, Dorrepaal E (2017) Winter warming effects on tundra shrub performance are species-specific and dependent on spring conditions. J Ecol. doi:10.1111/1365-2745.12872
Kuhry P, Dorrepaal E, Hugelius G, Schuur EAG, Tarnocai C (2010) Potential remobilization of belowground permafrost carbon under future global warming. Permafr Periglac 21:208–2014
Loya WM, Johnson LC, Kling GW, King JY, Reeburgh WS, Nadelhoffer KJ (2002) Pulse-labeling studies of carbon cycling in arctic tundra ecosystems: contribution of photosynthates to soil organic matter. Glob Biogeochem Cycles 16:1101
Makoto K, Klaminder J (2012) The influence of non-sorted circles on species diversity of vascular plants, bryophytes and lichens in Sub-Arctic Tundra. Polar Biol 35:1659–1667
Myers-Smith IH, Forbes BC, Wilmking M et al (2011) Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ Res Lett 6:045509
Nobrega S, Grogan P (2007) Deeper snow enhances winter respiration from both plant associated and bulk soil carbon pools in birch hummock tundra. Ecosystems 10:419–431
Olofsson J, Ericson L, Torp M, Stark S, Baxter R (2011) Carbon balance of Arctic tundra under increased snow cover mediated by a plant pathogen. Nat Clim Change 1:220–223
Parker TC, Subke J-A, Wookey PA (2015) Rapid carbon turnover beneath shrub and tree vegetation is associated with low soil carbon stocks at a subarctic treeline. Glob Change Biol 21:2070–2081
Pell M, Stenström J, Granhall U (2006) Soil respiration. In: Bloem J, Hopkins DW, Benedett A (eds) Microbial methods for assessing soil quality. CAB International, Wallingford, Oxfordshire
PP-systems: SRC-1/CPY-2/CPY-4 closed system chambers, for use with all EGM’s (1/2/3/4) and CIRAS-1, operator’s manual, version 3.34
Scheffer M, Carpenter SR (2003) Catastrophic regime shifts in ecosystem: linking theory to observation. Trend Ecol Evol 18:648–656
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Schimel JP, Bilbrough C, Welker JM (2004) Increased snow depth affects microbial activity and nitrogen mineralization in two Arctic tundra communities. Soil Biol Biochem 36:217–227
Schmidt SK, Lipson DA (2004) Microbial growth under the snow: implications for nutrient and allelochemical availability in temperate soils. Plant Soil 259:1–7
Schmidt SK, Costello EK, Nemergut DR, Cleveland CC, Reed SC, Weintraub MN, Meyer AF, Martin AM (2007) Biogeochemical consequences of rapid microbial turnover and seasonal succession in soil. Ecology 88:1379–1385
Semenchuk PR, Elberling B, Amtorp C, Winkler J, Rumpf S, Michelsen A, Cooper J (2015) Deeper snow alters soil nutrient availability and leaf nutrient status in high Arctic tundra. Biogeochemistry 124:81–94
Semenchuk PR, Christiansen CT, Grogan P, Elberling B, Cooper EJ (2016) Long-term experimentally deepened snow decreases growing-season respiration in a low- and high-arctic tundra ecosystem. J Geophys Res 121:1236–1248
Shaver GR, Chapin FSI (1995) Long-term responses to factorial NPK fertilizer treatment by Alaskan wet and moist tundra sedge species. Ecography 18:259–275
Sistla SA, Asao S, Schimel JP (2012) Detecting microbial N-limitation in tussock tundra soil: implications for Arctic soil organic carbon cycling. Soil Biol Biochem 55:78–84
Sturm M, McFadden JP, Liston GE, Chapin FS, Racine CH, Holmgren J (2001) Snow-shrub interactions in Arctic tundra: a hypothesis with climate implications. J Clim 14:336–344
Sturm M, Schimel JP, Michaelson G, Welker JM, Oberbauer SF, Liston GE, Fahnestock J, Romanovsky VE (2005) Winter biological processes could help convert arctic tundra to shrubland. Bioscience 55:17–26
Tan B, F-z Wu, W-q Yang, X-h He (2014) Snow removal alters soil microbial biomass and enzyme activity in a Tibetan alpine forest. Appl Soil Ecol 76:34–41
Tarnocai C, Canadell JG, Schuur EAG, Kuhry P, Mazhitova G, Zimov SA (2009) Soil organic carbon pools in the northern circumpolar permafrost region. Glob Biogeochem Cycle 23:GB2023
Walker D, Epstein HE, Gould WA et al (2004) Frost-boil ecosystems: complex interactions between landforms, soils, vegetation and climate. Permafr Periglac 15:171–188
Weedon JT, Kowalchuk GA, Aerts R, van Hal J, van Logtestijn R, Tas N, Röling WFM, van Bodegom PM (2012) Summer warming accelerates sub-arctic peatland nitrogen cycling without changing enzyme pools or microbial community structure. Glob Change Biol 18:138–150
Weintraub MN, Schimel JP (2005) Nitrogen cycling and the spread of shrubs control changes in the carbon balance of Arctic tundra ecosystems. Bioscience 5:408–415
Wheeler JA, Hoch G, Cortes AJ, Sedlacek J, Wipf S, Rixen C (2014) Increased spring freezing vulnerability for alpine shrubs under early snowmelt. Oecologia 175:219–229
Wipf S, Rixen C (2010) A review of snow manipulation experiments in Arctic and alpine tundra ecosystems. Polar Res 29:95–109
Ylänne H, Stark S, Tolvanen A (2015) Vegetation shift from deciduous to evergreen dwarf shrubs in response to selective herbivory offsets carbon losses: evidence from 19 years of warming and simulated herbivory in the subarctic tundra. Glob Change Biol 21:3696–3711
Acknowledgements
We thank Reiner Giesler and Johan Olofsson for logistic support, and Laurenz Teuber, Erik Lundin, Jacob DeKraai, Tuukka Mäkiranta, Marlene Kassel, Max Schuchardt, Jan Borgelt, Lea Fink and Jakob Eckstein for assistance in the field and laboratory. Jonatan Klaminder, Ann Milbau, Marina Becher, Gesche Blume-Werry, Frida Keuper and Kobayashi Makoto provided advice for the set-up of experimental design. This project was funded by Grants from Vetenskapsrådet (621-2011-5444), Formas (214-2011-788), and a Wallenberg Academy Fellowship (KAW 2012.0152) to ED.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Melany Fisk.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Väisänen, M., Krab, E.J. & Dorrepaal, E. Carbon dynamics at frost-patterned tundra driven by long-term vegetation change rather than by short-term non-growing season warming. Biogeochemistry 136, 103–117 (2017). https://doi.org/10.1007/s10533-017-0385-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-017-0385-y