Abstract
What causes the tissue-specific pathology of diseases resulting from mutations in housekeeping genes? Specifically, in spinocerebellar ataxia type 7 (SCA7), a neurodegenerative disorder caused by a CAG-repeat expansion in ATXN7 (which encodes an essential component of the mammalian transcription coactivation complex, STAGA), the factors underlying the characteristic progressive cerebellar and retinal degeneration in patients were unknown. We found that STAGA is required for the transcription initiation of miR-124, which in turn mediates the post-transcriptional cross-talk between lnc-SCA7, a conserved long noncoding RNA, and ATXN7 mRNA. In SCA7, mutations in ATXN7 disrupt these regulatory interactions and result in a neuron-specific increase in ATXN7 expression. Strikingly, in mice this increase is most prominent in the SCA7 disease-relevant tissues, namely the retina and cerebellum. Our results illustrate how noncoding RNA–mediated feedback regulation of a ubiquitously expressed housekeeping gene may contribute to specific neurodegeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
18 February 2015
In the version of this article initially published, Supplementary Figure 4k showed levels of mature miRNA-124 instead of miR-124 precursor. The error has been corrected in the Supplementary Text and Figures file and in the HTML and PDF versions of the article.
References
Gouw, L.G. et al. Analysis of the dynamic mutation in the SCA7 gene shows marked parental effects on CAG repeat transmission. Hum. Mol. Genet. 7, 525–532 (1998).
David, G. et al. Cloning of the SCA7 gene reveals a highly unstable CAG repeat expansion. Nat. Genet. 17, 65–70 (1997).
Gouw, L.G., Digre, K.B., Harris, C.P., Haines, J.H. & Ptacek, L.J., Autosomal dominant cerebellar ataxia with retinal degeneration: clinical, neuropathologic, and genetic analysis of a large kindred. Neurology 44, 1441–1447 (1994).
Holmberg, M. et al. Spinocerebellar ataxia type 7 (SCA7): a neurodegenerative disorder with neuronal intranuclear inclusions. Hum. Mol. Genet. 7, 913–918 (1998).
Helmlinger, D. et al. Ataxin-7 is a subunit of GCN5 histone acetyltransferase-containing complexes. Hum. Mol. Genet. 13, 1257–1265 (2004).
Cancel, G. et al. Distribution of ataxin-7 in normal human brain and retina. Brain 123, 2519–2530 (2000).
Mattick, J.S. The genetic signatures of noncoding RNAs. PLoS Genet. 5, e1000459 (2009).
Sayed, D. & Abdellatif, M. MicroRNAs in development and disease. Physiol. Rev. 91, 827–887 (2011).
Packer, A.N., Xing, Y., Harper, S.Q., Jones, L. & Davidson, B.L. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington's disease. J. Neurosci. 28, 14341–14346 (2008).
Johnson, R. & Buckley, N.J. Gene dysregulation in Huntington's disease: REST, microRNAs and beyond. Neuromolecular Med. 11, 183–199 (2009).
Lee, Y. et al. miR-19, miR-101 and miR-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nat. Neurosci. 11, 1137–1139 (2008).
Damiani, D. et al. Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina. J. Neuroscience 28, 4878–4887 (2008).
Schaefer, A. et al. Cerebellar neurodegeneration in the absence of microRNAs. J. Exp. Med. 204, 1553–1558 (2007).
Lagos-Quintana, M. et al. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 12, 735–739 (2002).
Sanuki, R. et al. miR-124a is required for hippocampal axogenesis and retinal cone survival through Lhx2 suppression. Nat. Neurosci. 14, 1125–1134 (2011).
Derrien, T. et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 22, 1775–1789 (2012).
Cabili, M.N. et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 25, 1915–1927 (2011).
Qureshi, I.A., Mattick, J.S. & Mehler, M.F. Long non-coding RNAs in nervous system function and disease. Brain Res. 1338, 20–35 (2010).
Koob, M.D. et al. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nat. Genet. 21, 379–384 (1999).
Daughters, R.S. et al. RNA gain-of-function in spinocerebellar ataxia type 8. PLoS Genet. 5 (2009).
La Spada, A.R. & Taylor, J.P. Repeat expansion disease: progress and puzzles in disease pathogenesis. Nat. Rev. Genet. 11, 247–258 (2010).
Faghihi, M.A. et al. Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol. 11 (2010).
Sopher, B.L. et al. CTCF regulates ataxin-7 expression through promotion of a convergently transcribed, antisense noncoding RNA. Neuron 70, 1071–1084 (2011).
Bithell, A., Johnson, R. & Buckley, N.J. Transcriptional dysregulation of coding and non-coding genes in cellular models of Huntington's disease. Biochem. Soc. Trans. 37, 1270–1275 (2009).
Mus, E., Hof, P.R. & Tiedge, H. Dendritic BC200 RNA in aging and in Alzheimer's disease. Proc. Natl. Acad. Sci. USA 104, 10679–10684 (2007).
Kumar, V. et al. Human disease-associated genetic variation impacts large intergenic non-coding RNA expression. PLoS Genet. 9, e1003201 (2013).
Tay, Y., Rinn, J. & Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 505, 344–352 (2014).
Nesterova, T.B. et al. Dicer regulates Xist promoter methylation in ES cells indirectly through transcriptional control of Dnmt3a. Epigenetics Chromatin 1, 2 (2008).
Visvanathan, J., Lee, S., Lee, B., Lee, J.W. & Lee, S.K. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 21, 744–749 (2007).
McMahon, S.J., Pray-Grant, M.G., Schieltz, D., Yates, J.R. & Grant, P.A. Polyglutamine-expanded spinocerebellar ataxia-7 protein disrupts normal SAGA and SLIK histone acetyltransferase activity. Proc. Natl. Acad. Sci. USA 102, 8478–8482 (2005).
Palhan, V.B. et al. Polyglutamine-expanded ataxin-7 inhibits STAGA histone acetyltransferase activity to produce retinal degeneration. Proc. Natl. Acad. Sci. USA 102, 8472–8477 (2005).
McCullough, S.D. et al. Reelin is a target of polyglutamine expanded ataxin-7 in human spinocerebellar ataxia type 7 (SCA7) astrocytes. Proc. Natl. Acad. Sci. USA 109, 21319–21324 (2012).
Chen, Y.C. et al. Gcn5 loss-of-function accelerates cerebellar and retinal degeneration in a SCA7 mouse model. Hum. Mol. Genet. 21, 394–405 (2012).
Yoo, S.Y. et al. SCA7 knockin mice model human SCA7 and reveal gradual accumulation of mutant ataxin-7 in neurons and abnormalities in short-term plasticity. Neuron 37, 383–401 (2003).
Karginov, F.V. et al. A biochemical approach to identifying microRNA targets. Proc. Natl. Acad. Sci. USA 104, 19291–19296 (2007).
Agirre, X. et al. Epigenetic silencing of the tumor suppressor microRNA Hsa-miR-124a regulates CDK6 expression and confers a poor prognosis in acute lymphoblastic leukemia. Cancer Res. 69, 4443–4453 (2009).
Yoo, A.S., Staahl, B.T., Chen, L. & Crabtree, G.R. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature 460, 642–646 (2009).
Makeyev, E.V., Zhang, J., Carrasco, M.A. & Maniatis, T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell 27, 435–448 (2007).
Liu, X.S. et al. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS ONE 6 (2011).
Shi, X.B. et al. Tumor suppressive miR-124 targets androgen receptor and inhibits proliferation of prostate cancer cells. Oncogene 32, 4130–4138 (2013).
Xia, H. et al. Loss of brain-enriched miR-124 microRNA enhances stem-like traits and invasiveness of glioma cells. J. Biol. Chem. 287, 9962–9971 (2012).
Hendrickson, D.G., Hogan, D.J., Herschlag, D., Ferrell, J.E. & Brown, P.O. Systematic identification of mRNAs recruited to argonaute 2 by specific microRNAs and corresponding changes in transcript abundance. PLoS ONE 3, e2126 (2008).
Fang, M. et al. The miR-124 regulates the expression of BACE1/β-secretase correlated with cell death in Alzheimer's disease. Toxicol. Lett. 209, 94–105 (2012).
Nakamachi, Y. et al. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 60, 1294–1304 (2009).
Zander, C. et al. Similarities between spinocerebellar ataxia type 7 (SCA7) cell models and human brain: proteins recruited in inclusions and activation of caspase-3. Hum. Mol. Genet. 10, 2569–2579 (2001).
Bandiera, S. et al. Genetic variations creating microRNA target sites in the FXN 3′-UTR affect frataxin expression in Friedreich ataxia. PLoS ONE 8, e54791 (2013).
Chou, A.H. et al. Polyglutamine-expanded ataxin-7 causes cerebellar dysfunction by inducing transcriptional dysregulation. Neurochem. Int. 56, 329–339 (2010).
Abou-Sleymane, G. et al. Polyglutamine expansion causes neurodegeneration by altering the neuronal differentiation program. Hum. Mol. Genet. 15, 691–703 (2006).
Rajakulendran, S. et al. Deletion of chromosome 12q21 affecting KCNC2 and ATXN7L3B in a family with neurodevelopmental delay and ataxia. J. Neurol. Neurosurg. Psychiatry 84, 1225–1257 (2013).
Su, A.I. et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA 101, 6062–6067 (2004).
Yuan, B., Latek, R., Hossbach, M., Tuschl, T. & Lewitter, F. siRNA Selection Server: an automated siRNA oligonucleotide prediction server. Nucleic Acids Res. 32, W130–W134 (2004).
Nesterova, T.B. et al. Dicer regulates Xist promoter methylation in ES cells indirectly through transcriptional control of Dnmt3a. Epigenetics Chromatin 1 (2008).
Lane, L. et al. neXtProt: a knowledge platform for human proteins. Nucleic Acids Res. 40, D76–D83 (2012).
Betel, D., Wilson, M., Gabow, A., Marks, D.S. & Sander, C. The microRNA.org resource: targets and expression. Nucleic Acids Res. 36, D149–D153 (2008).
Krzywinski, M. et al. Circos: an information aesthetic for comparative genomics. Genome Res. 19, 1639–1645 (2009).
Geiss, G.K. et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotechnol. 26, 317–325 (2008).
Brumbaugh, C.D., Kim, H.J., Giovacchini, M. & Pourmand, N. NanoStriDE: normalization and differential expression analysis of NanoString nCounter data. BMC Bioinformatics 12, 479 (2011).
Myers, R.M. et al. A user's guide to the Encyclopedia of DNA elements (ENCODE). PLoS Biol. 9 (2011).
Chen, Y.C. et al. Gcn5 loss-of-function accelerates cerebellar and retinal degeneration in a SCA7 mouse model. Hum. Mol. Genet. 21, 394–405 (2012).
Yoo, S.Y. et al. SCA7 knockin mice model human SCA7 and reveal gradual accumulation of mutant ataxin-7 in neurons and abnormalities in short-term plasticity. Neuron 37, 383–401 (2003).
Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals (National Academy Press, 1996).
Chodroff, R.A. et al. Long noncoding RNA genes: conservation of sequence and brain expression among diverse amniotes. Genome Biol. 11, R72 (2010).
Deo, M., Yu, J.Y., Chung, K.H., Tippens, M. & Turner, D.L. Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Dev. Dyn. 235, 2538–2548 (2006).
Acknowledgements
We thank E. Becker (University of Oxford) for vectors, helpful discussions and comments on the manuscript; A. Barnard, M. McClements and R. MacLaren (all at John Radcliffe Hospital, University of Oxford) for WERI cells; members of the A.C.M. and C.P.P. laboratories for insightful comments and suggestions; I. Baumgarten for valuable discussions and establishing patient fibroblast cultures; and H.Y. Zoghbi for the SCA7100Q/5Q mice . This work was supported by funding from the Medical Research Council (to C.P.P. and Weatherall Institute of Molecular Medicine (WIMM) Strategic Award, MRC G0902418, to B.R.S. and T.A.F.), a Marie Curie Intra-European Career Development Award (to A.C.M.), the University of Oxford (to A.C.M.), the Royal Society (to A.C.M.), a European Research Council Advanced Grant (to C.P.P., A.C.M., K.W.V. and T.S.), the French National Research Foundation (to S.A.), the South African National Research Foundation (to L.M.W.), the Medical Research Council (to S.A. and L.M.W.), the University of Cape Town (to L.M.W.), the Harry Crossley Foundation (to L.M.W.), the Commonwealth Scholarship Commission (to L.M.W.), the Clarendon Fund (to J.Y.T.), the Natural Sciences Engineering Research Council of Canada (to J.Y.T.), the Wellcome Trust (WT081385 to S.C., T.N. and N.B.), a European Research Council Starting Grant (to P.L.O.), Ataxia UK (to H.J.C. and M.A.V.), the French Association against Myopathies (AFM) (to A.B. and long-term fellowship to S.A.), the Association Connaître les Syndrômes Cérébelleux (to A.S. and S.A.) and a French Ministry of Research fellowship (to M.M.).
Author information
Authors and Affiliations
Contributions
A.C.M. conceived the study; J.Y.T. performed experiments and analyzed results with contributions from K.W.V., M.A.V., T.S., L.M.W., H.J.C., M.M., S.A., B.R.S., S.C., T.N. and P.L.O.; N.B., T.A.F., A.B., A.S., M.J.W., C.P.P. and A.C.M. supervised the analysis; C.P.P. and A.C.M. supervised the study; J.Y.T., C.P.P. and A.C.M. wrote the manuscript. All authors read, contributed to and agreed with the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
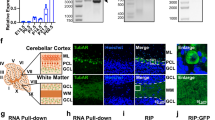
Supplementary Figure 1 The expression of lnc-SCA7, a retropseudogene of Atxn7l3, is correlated with Atxn7 expression.
(A and B) Correlation between the expression of lnc-SCA7 (x-axis) and ATXN7 (y-axis) measured by quantitative reverse transcription PCR (qRT-PCR) [cross-threshold cycle (CT)] using transcript specific primers (Supplementary Table 4) in (A) 20 adult human tissues (adipose, bladder, brain, cervix, colon, esophagus, heart, kidney, liver, lung, ovary, placenta, prostate, skeletal muscle, small intestine, spleen, testes, thymus, thyroid, and trachea) and (B) across 11 mouse adult tissues (bladder, brain, colon, heart, kidney, liver, lung, pancreas, skeletal muscle, small intestine, and stomach) and 9 brain regions (retina, cerebellum, cortex, entorhinal cortex, hippocampus, hypothalamus, medulla, olfactory bulb, and striatum). The correlation is stronger across central nervous system regions (R2 = 0.94, blue) than in non-CNS tissues (R2 = 0.69, red). (C) Pairwise sequence alignment between the open reading frame (ORF, blue) of mouse Atxn7l3 and the homologous region in lnc-SCA7. A frame-shifting deletion (black box) in lnc-SCA7 resulted in a premature stop codon (red box). The putatively coding region conserved between lnc-SCA7 and Atxn7l3 that was used to raise a custom antibody recognizing both Atxn7l3 and the putative peptide encoded by lnc-SCA7 is highlighted in red. The lnc-SCA7-STOP recombinant locus was generated by creating a premature stop codon in lnc-SCA7 by site-directed single nucleotide mutagenesis at nucleotide position 14 (C to A). Insertions and deletions in the pairwise alignment are represented by dashes. (D) Schematic diagram representing the protein domains within ATXN7L3 (ATXN7L3_HUMAN; Q14CW9) and lnc-SCA7 (ATXN7L3B) obtained from Pfam17. The putative protein product of lnc-SCA7 (97 amino acids) is shorter than ATXN7L3 (354 amino acids) and lacks the protein domains present in ATXN7L3, namely transcriptional regulation (Sgf11, green) and Zinc-binding (SCA7, red) domains. (E) Expression levels [cross-threshold cycle (CT)] of lnc-SCA7 (blue) and Atxn7l3 (grey) in N2A cells. Expression levels for the 2 genes were normalized relative to Gapdh. (F) In mouse neuroblastoma cells (N2A) whole cell protein lysate (left), the custom antibody raised against a conserved region between lnc-SCA7 and ATXN7L3 detected, by western blot, only a band at approximately 38kDa, likely to be Atxn7l3 (predicted size 39kDa, arrow). No detectable band was apparent at the expected size for lnc-SCA7 ORF (predicted size of 11kDa), suggesting that lnc-SCA7 is not translated into a stable protein (right).
Supplementary Figure 2 Knockdown of cytoplasmic lnc-SCA7.
(A) Sequence alignment between regions in mouse Atxn7l3 and lnc-SCA7 used to design specific short hairpin RNAs (shRNAs) against lnc-SCA7. The initial base of the predicted binding sites in these transcripts is noted to the left of the alignment. Identical nucleotides between Atxn7l3 and lnc-SCA7 are denoted by vertical lines. Regions targeted by the siRNAs are highlighted in blue. (B) Expression levels of lnc-SCA7 (blue) and Atxn7 (red) following knockdown of lnc-SCA7 in N2A cells using each of the 3 designed shRNAs (sh-lnc-SCA7, sh-lnc-SCA7_1, and sh-lnc-SCA7_2). (C) lnc-SCA7 (blue) and Atxn7 mRNA (red) are found predominantly in the cytoplasm of N2A cells (y-axis). Malat1 (light grey), a known nuclear transcript, was used as control. Fold enrichments (expression level measured in the cytoplasmic relative to the expression level measured in the nucleus) for all genes were measured and normalized against that of Gapdh, a cytoplasmic single exonic transcript. Error bars represent s.d.m. n = 3 cell cultures per condition; * p < 0.05; ** p < 0.01; Two-tailed Student’s t-test.
Supplementary Figure 3 The ability of lnc-SCA7 to regulate Atxn7 expression is dependent on miR-124 but not on miR-16.
Sequence alignment of (A) miR-124 and (B) miR-16 and their respective miRNA response elements (MREs) within the 3’ UTRs of mouse lnc-SCA7 (top panels) or Atxn7 (bottom panels). The initial base of the predicted binding sites in these transcripts is noted to the left of the alignment. Identical nucleotides between miR-124/miR-16 with lnc-SCA7 and Atxn7 are denoted by vertical lines. (C) Effect of over-expression of miR-16 mimics (top right box, light blue) in N2A cells on the expression levels of lnc-SCA7 (NS, blue) or Atxn7 (NS, red) relative to a non-specific sequence used as control (white). (D) miR-16 (light blue) is relatively more lowly expressed in N2A cells compared to miR-124 (dark grey). Error bars represent s.d.m.; n = 3 cell cultures per condition; *** p < 0.001; Two-tailed Student’s t-test.
Supplementary Figure 4 lnc-SCA7 modulates miR-124 abundance in mouse and human neuroblastoma cells.
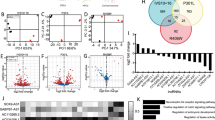
(A) Effect on pri-miR-124a_1 (light grey, AK044422) abundance over the time course of 72h of knockdown of lnc-SCA7 (blue) in N2A cells. (B) Effect, relative to control (white), of lnc-SCA7 knockdown in human neuroblastoma cells (SH-SY5Y), on pri-miR-124a (blue) and ATXN7 (red) expression. (C) Transfection of miR-124 inhibitors in SH-SY5Y cells (top-right insert, dark grey) results in increased levels of both lnc-SCA7 (blue) and Atxn7 (red) relative to a negative miRNA transfection control (white). Fold enrichment/depletion in expression is calculated relative to transcript abundance following transfection with the scrambled control. Genome browser views of regions of the mouse genome (mm9) encoding the (D) negative control region and pri-miR-124-1, (E) pri-miR-124-2 and (F) pri-miR-124-3 putative promoter regions. These regions are hypersensitive to DNase I treatment (grey, y-axis peak height correlates with number of ChIP-seq DHSI reads) in the cerebellum. Regions enriched in the cerebellum for H3K27ac (green) were defined as putative promoters: miR-124-1 (chr14:65,205,705 – 65,207,200), miR-124-2 (chr3:17,694,143 – 17,695,600), miR-124-3 (chr2:180,627,439 – 180,628,900) and negative control region (chr14:65,183,839 – 65,185,271). Red arrows indicate the position of primers pairs used to test regions enriched in STAGA binding: 1b, 1a, 2a, 2b, 2c, 3d, 3c, 3b, 3a, na, and nb (Supplementary Table 4). (G) Transfection of any of the 3 miR-124-prom-luc (dark grey) in N2A cells resulted in significantly increased normalized reporter activity relative to constructs for which these regions were cloned in the antisense orientation (white). No significant (NS) change was observed for the negative control region. (H) ChIP-qPCR revealed significantly decreased enrichment in GCN5 binding at miR-124 promoters in N2As with stably knocked down lnc-SCA7 (blue) relative to scramble control (white). (I) In N2As, and relative to scrambled knockdown transfection control (white), co-transfection of sh-lnc-SCA7 with recombinant luciferase constructs containing the pri-miR-124 promoter regions resulted in significant decrease in normalized reporter activities for all pri-miR-124 promoter regions (dark grey). No depletion was found in the negative control region, NC-prom-luc. n = 3 biological replicates per condition. (J) In N2As, and relative to Atxn7-MUT (light grey), co-transfection of Atxn7-WT with recombinant luciferase constructs containing the pri-miR-124 promoter regions resulted in the significant increase in normalized reporter activities for all pri-miR-124 promoter regions (dark grey). (K) Over-expression of Atxn7-WT significantly reduced abundance of mature miR-124 (dark grey) relative to control (white) and Atxn7-MUT (light-grey). Error bars represent s.d.m. n = 3 cell cultures per condition; * p < 0.05; ** p < 0.01; ***; p < 0.001; Not significant, NS; Two-tailed Student’s t-test.
Supplementary Figure 5 miR-124 expression in human and mouse adult tissues.
miR-124 expression level (y-axis) [(cross-threshold cycle (CT)] relative to Gapdh/GAPDH measured by qRT-PCR in (A) 22 human tissues, including a human fibroblast cell line derived from healthy human controls, and WERI retinoblastoma cells, and (B) 9 mouse CNS tissues, whole brain, and 10 mouse non-CNS tissues. Error bars represent s.d.m.; n = 3 biological replicates per condition; *** p < 0.001; Two-tailed Student’s t-test.
Supplementary Figure 6 Decreases in miR-124 levels are associated with increased ATXN7 and lnc-SCA7 expression in human fibroblasts.
Transfection of miR-124 inhibitors in human fibroblast cells (top-right insert, dark grey) results in increased levels of both lnc-SCA7 (blue) and Atxn7 (red) relative to a negative miRNA transfection control (white). Error bars represent s.d.m. n = 3 cell cultures per condition; * p < 0.05; ** p < 0.01; *** p < 0.001; Not significant, NS; Two-tailed Student’s t-test.
.
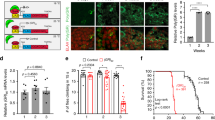
Supplementary Figure 7 Expression levels of Atxn7, lnc-SCA7, miR-124 and targets in SCA7 mice.
Fold difference in normalized expression (relative to Gadph) measured by qRT-PCR of miR-124 (dark grey), lnc-SCA7 (blue) and ATXN7 (red) across tissues derived from SCA7 mouse models relative to matched controls (white): (A) Retina and (B) cerebellum of 28 weeks SCA7100Q/100Q mice, and (C) retina, (D) cerebellum, (E) cortex, (F) striatum, (G) olfactory bulb and (H) spinal cord of SCA7266Q/5Q animals. (I and J) The fold-difference (y-axis) in expression in the (I) cerebellum or (J) retina of 5 week SCA7266Q/5Q mice (grey) relative to matched littermate SCA75Q/5Q control mice (white) were elevated for 8 and 12 (of 13) known miR-124 targets (light grey), respectively. Relative pri-miR-124a1 (dark grey) levels were reduced in these tissues in SCA7 mice. Fold difference in normalized expression (relative to Gadph) measured by qRT-PCR of miR-124 (dark grey), lnc-SCA7 (blue) and ATXN7 (red) across tissues derived from two SCA7 mouse models relative to controls. (K, L) livers, (M, N) lungs and (O) muscle of 28 week SCA7100Q/100Q or 5 week SCA7266Q/5Q mice relative to matched control mice (SCA75Q/5Q, white). Error bars represent s.d.m. n = 3 cell cultures per condition; * p < 0.05; ** p < 0.01; ***,p < 0.001; Not significant, NS; Two-tailed Student’s t-test.
Supplementary Figure 8 Expression levels of Atxn7 and lnc-SCA7 measured with digital droplet PCR correlate with standard qRT-PCR measurements.
Expression levels of (A) lnc-SCA7 (blue) and (B) Atxn7 (red) quantified using ddPCR (y-axis, copy number per μL of cDNA) and qRT-PCR (x-axis, cycle threshold) in the cerebellum, lung, and liver of 5 week SCA7266Q/5Q and that of matched littermate SCA75Q/5Q control mice (white) are strongly correlated. n = 3 biological replicates per condition. Error bars represent s.d.m.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Note (PDF 4446 kb)
Supplementary Data Set 1
Uncropped image of western blots used in the main text (PDF 459 kb)
Supplementary Table 1
Prediction of MREs for miR-124 for all STAGA-encoding genes in both mouse and human (microrna.org, all miRSVR scores) (XLSX 14 kb)
Supplementary Table 2
Genome-wide microRNA expression levels quantified by NanoString following the knockdown of lnc-SCA7 compared to scramble control, the over-expression of wild-type lnc-SCA7 and the over-expression of mutant lnc-SCA7 with mutated miR-124 MREs compared to pcDNA3.1(+) empty vector control in N2A cells (XLSX 255 kb)
Supplementary Table 3
Absolute quantification (in copy number per μL of cDNA) measured by digital droplet PCR (ddPCR) of lnc-SCA7 and Atxn7 in N2A and ES cells, as well as the cerebellum, lung, and liver of SCA7266Q/5Q and their matched littermate SCA75Q/5Q control mice (XLSX 10 kb)
Supplementary Table 4
Sequence of oligos used in the study (XLSX 72 kb)
Rights and permissions
About this article
Cite this article
Tan, J., Vance, K., Varela, M. et al. Cross-talking noncoding RNAs contribute to cell-specific neurodegeneration in SCA7. Nat Struct Mol Biol 21, 955–961 (2014). https://doi.org/10.1038/nsmb.2902
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2902
This article is cited by
-
Loss-of-function variants affecting the STAGA complex component SUPT7L cause a developmental disorder with generalized lipodystrophy
Human Genetics (2024)
-
The landscape of the long non-coding RNAs in developing mouse retinas
BMC Genomics (2023)
-
Functional implications of paralog genes in polyglutamine spinocerebellar ataxias
Human Genetics (2023)
-
Molecular landscape of long noncoding RNAs in brain disorders
Molecular Psychiatry (2021)
-
Nrn1 Overexpression Attenuates Retinal Ganglion Cell Apoptosis, Promotes Axonal Regeneration, and Improves Visual Function Following Optic Nerve Crush in Rats
Journal of Molecular Neuroscience (2021)