Abstract
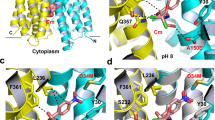
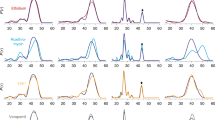
Multidrug antiporters of the major facilitator superfamily couple proton translocation to the extrusion of cytotoxic molecules. The conformational changes that underlie the transport cycle and the structural basis of coupling of these transporters have not been elucidated. Here we used extensive double electron-electron resonance measurements to uncover the conformational equilibrium of LmrP, a multidrug transporter from Lactococcus lactis, and to investigate how protons and ligands shift this equilibrium to enable transport. We find that the transporter switches between outward-open and outward-closed conformations, depending on the protonation states of specific acidic residues forming a transmembrane protonation relay. Our data can be framed in a model of transport wherein substrate binding initiates the transport cycle by opening the extracellular side. Subsequent protonation of membrane-embedded acidic residues induces substrate release to the extracellular side and triggers a cascade of conformational changes that concludes in proton release to the intracellular side.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Li, X.-Z. & Nikaido, H. Efflux-mediated drug resistance in bacteria: an update. Drugs 69, 1555–1623 (2009).
Putman, M., van Veen, H.W., Degener, J.E. & Konings, W.N. The lactococcal secondary multidrug transporter LmrP confers resistance to lincosamides, macrolides, streptogramins and tetracyclines. Microbiology 147, 2873–2880 (2001).
Jardetzky, O. Simple allosteric model for membrane pumps. Nature 211, 969–970 (1966).
Tanford, C. Mechanism of free energy coupling in active transport. Annu. Rev. Biochem. 52, 379–409 (1983).
Forrest, L.R., Krämer, R. & Ziegler, C. The structural basis of secondary active transport mechanisms. Biochim. Biophys. Acta 1807, 167–188 (2011).
Reddy, V.S., Shlykov, M.A., Castillo, R., Sun, E.I. & Saier, M.H. The major facilitator superfamily (MFS) revisited. FEBS J. 279, 2022–2035 (2012).
Huang, Y., Lemieux, M.J., Song, J., Auer, M. & Wang, D.-N. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science 301, 616–620 (2003).
Guan, L., Mirza, O., Verner, G., Iwata, S. & Kaback, H.R. Structural determination of wild-type lactose permease. Proc. Natl. Acad. Sci. USA 104, 15294–15298 (2007).
Dang, S. et al. Structure of a fucose transporter in an outward-open conformation. Nature 467, 734–738 (2010).
Newstead, S. et al. Crystal structure of a prokaryotic homologue of the mammalian oligopeptide-proton symporters, PepT1 and PepT2. EMBO J. 30, 417–426 (2011).
Yin, Y., He, X., Szewczyk, P., Nguyen, T. & Chang, G. Structure of the multidrug transporter EmrD from Escherichia coli. Science 312, 741–744 (2006).
Sun, L. et al. Crystal structure of a bacterial homologue of glucose transporters GLUT1–4. Nature 490, 361–366 (2012).
Lemieux, M.J., Huang, Y. & Wang, D.-N. The structural basis of substrate translocation by the Escherichia coli glycerol-3-phosphate transporter: a member of the major facilitator superfamily. Curr. Opin. Struct. Biol. 14, 405–412 (2004).
Holyoake, J. & Sansom, M.S.P. Conformational change in an MFS protein: MD simulations of LacY. Structure 15, 873–884 (2007).
Enkavi, G. & Tajkhorshid, E. Simulation of spontaneous substrate binding revealing the binding pathway and mechanism and initial conformational response of GlpT. Biochemistry 49, 1105–1114 (2010).
Liu, Z., Madej, M.G. & Kaback, H.R. Helix dynamics in LacY: helices II and IV. J. Mol. Biol. 396, 617–626 (2010).
Zhou, Y., Madej, M.G., Guan, L., Nie, Y. & Kaback, H.R. An early event in the transport mechanism of LacY protein: interaction between helices V and I. J. Biol. Chem. 286, 30415–30422 (2011).
Kaback, H.R., Smirnova, I., Kasho, V., Nie, Y. & Zhou, Y. The alternating access transport mechanism in LacY. J. Membr. Biol. 239, 85–93 (2011).
Smirnova, I., Kasho, V., Sugihara, J., Vázquez-Ibar, J.L. & Kaback, H.R. Role of protons in sugar binding to LacY. Proc. Natl. Acad. Sci. USA 109, 16835–16840 (2012).
Yin, Y., Jensen, M.Ø., Tajkhorshid, E. & Schulten, K. Sugar binding and protein conformational changes in lactose permease. Biophys. J. 91, 3972–3985 (2006).
Smirnova, I. et al. Sugar binding induces an outward facing conformation of LacY. Proc. Natl. Acad. Sci. USA 104, 16504–16509 (2007).
Guan, L. & Kaback, H.R. Binding affinity of lactose permease is not altered by the H+ electrochemical gradient. Proc. Natl. Acad. Sci. USA 101, 12148–12152 (2004).
Fluman, N., Ryan, C.M., Whitelegge, J.P. & Bibi, E. Dissection of mechanistic principles of a secondary multidrug efflux protein. Mol. Cell 47, 777–787 (2012).
Mazurkiewicz, P., Driessen, A.J.M. & Konings, W.N. Energetics of wild-type and mutant multidrug resistance secondary transporter LmrP of Lactococcus lactis. Biochim. Biophys. Acta 1658, 252–261 (2004).
Lewinson, O. et al. The Escherichia coli multidrug transporter MdfA catalyzes both electrogenic and electroneutral transport reactions. Proc. Natl. Acad. Sci. USA 100, 1667–1672 (2003).
Bolhuis, H. et al. Energetics and mechanism of drug transport mediated by the lactococcal multidrug transporter LmrP. J. Biol. Chem. 271, 24123–24128 (1996).
Jeschke, G. DEER distance measurements on proteins. Annu. Rev. Phys. Chem. 63, 419–446 (2012).
McHaourab, H.S., Steed, P.R. & Kazmier, K. Toward the fourth dimension of membrane protein structure: insight into dynamics from spin-labeling EPR spectroscopy. Structure 19, 1549–1561 (2011).
Zou, P. & McHaourab, H.S. Increased sensitivity and extended range of distance measurements in spin-labeled membrane proteins: Q-band double electron-electron resonance and nanoscale bilayers. Biophys. J. 98, L18–L20 (2010).
Putman, M., Koole, L.A., van Veen, H.W. & Konings, W.N. The secondary multidrug transporter LmrP contains multiple drug interaction sites. Biochemistry 38, 13900–13905 (1999).
Mazurkiewicz, P., Konings, W.N. & Poelarends, G.J. Acidic residues in the lactococcal multidrug efflux pump LmrP play critical roles in transport of lipophilic cationic compounds. J. Biol. Chem. 277, 26081–26088 (2002).
Andersen, A.Z. et al. The metabolic pH response in Lactococcus lactis: an integrative experimental and modelling approach. Comput. Biol. Chem. 33, 71–83 (2009).
Hakizimana, P., Masureel, M., Gbaguidi, B., Ruysschaert, J.-M. & Govaerts, C. Interactions between phosphatidylethanolamine headgroup and LmrP, a multidrug transporter: a conserved mechanism for proton gradient sensing? J. Biol. Chem. 283, 9369–9376 (2008).
Seol, W. & Shatkin, A.J. Site-directed mutants of Escherichia coli α-ketoglutarate permease (KgtP). Biochemistry 31, 3550–3554 (1992).
Pazdernik, N.J., Matzke, E.A., Jessen-Marshall, A.E. & Brooker, R.J. Roles of charged residues in the conserved motif, G-X-X-X-D/E-R/K-X-G-[X]-R/K-R/K, of the lactose permease of Escherichia coli. J. Membr. Biol. 174, 31–40 (2000).
Yamaguchi, A., Someya, Y. & Sawai, T. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by transposon Tn10. The role of a conserved sequence motif, GXXXXRXGRR, in a putative cytoplasmic loop between helices 2 and 3. J. Biol. Chem. 267, 19155–19162 (1992).
Schaedler, T.A. & van Veen, H.W. A flexible cation binding site in the multidrug major facilitator superfamily transporter LmrP is associated with variable proton coupling. FASEB J. 24, 3653–3661 (2010).
Gbaguidi, B. et al. Proton motive force mediates a reorientation of the cytosolic domains of the multidrug transporter LmrP. Cell Mol. Life Sci. 61, 2646–2657 (2004).
Gbaguidi, B., Hakizimana, P., Vandenbussche, G. & Ruysschaert, J.-M. Conformational changes in a bacterial multidrug transporter are phosphatidylethanolamine-dependent. Cell Mol. Life Sci. 64, 1571–1582 (2007).
Mazurkiewicz, P., Poelarends, G.J., Driessen, A.J.M. & Konings, W.N. Facilitated drug influx by an energy-uncoupled secondary multidrug transporter. J. Biol. Chem. 279, 103–108 (2004).
Madej, M.G., Soro, S.N. & Kaback, H.R. Apo-intermediate in the transport cycle of lactose permease (LacY). Proc. Natl. Acad. Sci. USA 109, E2970–E2978 (2012).
Hirai, T. & Subramaniam, S. Structure and transport mechanism of the bacterial oxalate transporter OxlT. Biophys. J. 87, 3600–3607 (2004).
Bolhuis, H. et al. Multidrug resistance in Lactococcus lactis: evidence for ATP-dependent drug extrusion from the inner leaflet of the cytoplasmic membrane. EMBO J. 15, 4239–4245 (1996).
Shapiro, A.B. & Ling, V. Extraction of Hoechst 33342 from the cytoplasmic leaflet of the plasma membrane by P-glycoprotein. Eur. J. Biochem. 250, 122–129 (1997).
Mitchell, B.A., Paulsen, I.T., Brown, M.H. & Skurray, R.A. Bioenergetics of the staphylococcal multidrug export protein QacA. Identification of distinct binding sites for monovalent and divalent cations. J. Biol. Chem. 274, 3541–3548 (1999).
Ocaktan, A., Yoneyama, H. & Nakae, T. Use of fluorescence probes to monitor function of the subunit proteins of the MexA-MexB-oprM drug extrusion machinery in Pseudomonas aeruginosa. J. Biol. Chem. 272, 21964–21969 (1997).
Baker, J., Wright, S.H. & Tama, F. Simulations of substrate transport in the multidrug transporter EmrD. Proteins 80, 1620–1632 (2012).
Frillingos, S. & Kaback, H.R. The role of helix VIII in the lactose permease of Escherichia coli: II. Site-directed sulfhydryl modification. Protein Sci. 6, 438–443 (1997).
Soskine, M., Adam, Y. & Schuldiner, S. Direct evidence for substrate-induced proton release in detergent-solubilized EmrE, a multidrug transporter. J. Biol. Chem. 279, 9951–9955 (2004).
Lu, M. et al. Structures of a Na+-coupled, substrate-bound MATE multidrug transporter. Proc. Natl. Acad. Sci. USA 110, 2099–2104 (2013).
Thompson, J.D., Higgins, D.G. & Gibson, T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Eswar, N. et al. Comparative protein structure modeling using MODELLER. Curr. Protoc. Protein Sci. Chapter 2 Unit 2.9 (2007).
Pettersen, E.F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Putman, M., van Veen, H.W., Poolman, B. & Konings, W.N. Restrictive use of detergents in the functional reconstitution of the secondary multidrug transporter LmrP. Biochemistry 38, 1002–1008 (1999).
Jeschke, G. Distance measurements in the nanometer range by pulse EPR. ChemPhysChem 3, 927–932 (2002).
Jeschke, G. et al. DeerAnalysis2006—a comprehensive software package for analyzing pulsed ELDOR data. Appl. Magn. Reson. 30, 473–498 (2006).
Chiang, Y.-W., Borbat, P.P. & Freed, J.H. The determination of pair distance distributions by pulsed ESR using Tikhonov regularization. J. Magn. Reson. 172, 279–295 (2005).
Acknowledgements
We thank H. Remaut for insightful discussions and H. Koteiche and R. Steed for critically reading the manuscript. This work was supported by a Mandat d'Impulsion Scientifique (no. F.4523.12) from the Fonds de la Recherche Scientifique (FRS-FNRS), Belgium, to C.G. and by the National Institute for General Medical Sciences–US National Institutes of Health (GM-077659) to H.S.M. M.M. was a research fellow of the Fonds pour la Formation à la Recherche dans l′Industrie et dans l′Agriculture (FRIA), Belgium, and the FRS-FNRS. C.M. is a research fellow of the FRIA. C.G. is a research associate of the FRS-FNRS.
Author information
Authors and Affiliations
Contributions
M.M., C.M., H.S.M. and C.G. were involved in experimental design. M.M. and C.M. performed mutagenesis, expression, activity, purification and labeling experiments. M.M., R.A.S., S.M. and H.S.M. performed EPR measurements. R.A.S. and H.M. performed DEER and continuous-wave data analysis. C.G. and C.M. performed molecular modeling. C.G. and H.M. oversaw all aspects of the experiments and manuscript preparation. All authors participated in interpreting the data and writing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results and Supplementary Figures 1–13. (PDF 3414 kb)
Rights and permissions
About this article
Cite this article
Masureel, M., Martens, C., Stein, R. et al. Protonation drives the conformational switch in the multidrug transporter LmrP. Nat Chem Biol 10, 149–155 (2014). https://doi.org/10.1038/nchembio.1408
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1408
This article is cited by
-
Dynamics of efflux pumps in antimicrobial resistance, persistence, and community living of Vibrionaceae
Archives of Microbiology (2024)
-
Molecular mechanism of sugar transport in plants unveiled by structures of glucose/H+ symporter STP10
Nature Plants (2021)
-
Structure and mechanism of a redesigned multidrug transporter from the Major Facilitator Superfamily
Scientific Reports (2020)
-
Complexities of a protonatable substrate in measurements of Hoechst 33342 transport by multidrug transporter LmrP
Scientific Reports (2020)
-
Hydrogen-deuterium exchange mass spectrometry captures distinct dynamics upon substrate and inhibitor binding to a transporter
Nature Communications (2020)