Abstract
Recent studies of the genetic structure of stream-dwelling organisms have suggested that fine-scale patterns are the consequence of patchy recruitment from a small number of matings and limited in-stream dispersal. Predictions of this hypothesis were tested by spatial and temporal analysis of the genetic structure of populations of a stream mayfly (Bungona sp.: Baetidae) in subtropical streams in south-eastern Queensland. Significant departures from Hardy–Weinberg proportions occurred more often than would be predicted by chance alone and no consistent pattern was observed across sites, loci or sampling times. As in previous studies, the largest differentiation was observed at the smallest spatial scale (reaches within streams) on most sampling occasions. These data provide additional support for a patchy recruitment hypothesis. Despite the fine-scale population structure, there was evidence of widespread adult dispersal across the study region, especially between streams and subcatchments within the same block of continuous dense forest.
Similar content being viewed by others
Introduction
Analysis of the patterns and levels of genetic differentiation among populations of aquatic organisms has recently been used to infer the mechanisms and extent of dispersal (e.g. Rank, 1992; Hughes et al., 1995; Gibbs et al., 1998). This approach assumes that there is a close relationship between gene flow and genetic differentiation, such that high levels of gene flow result in genetic homogeneity and limited gene flow results in genetic differentiation due to genetic drift and/or natural selection. Some studies have tested these ideas empirically. For example, Doherty et al. (1996) showed a negative relationship between length of larval life, and thus potential for gene flow, and the degree of genetic differentiation for seven species of fish on the Great Barrier Reef. Waples (1987) showed a similar relationship for inshore fishes in the Pacific.
Given that effective dispersal is likely to decline with distance, one would also expect that the level of genetic differentiation would increase as the spatial scale examined is increased. This pattern has been shown in many terrestrial species (e.g. beetles, Rank, 1992) and in freshwater shrimps restricted to stream channels (Hughes et al., 1995, 1996). However, many studies of marine species with planktonic larvae and presumably widespread dispersal have reported significant genetic variation on very small spatial scales. Johnson & Black (1984) reported significant genetic differentiation among samples of an intertidal limpet from adjacent rock platforms on the same rocky shore. Similar heterogeneity has been reported for other marine species, for example, bivalve molluscs (David et al., 1997) and coral reef fishes (Doherty et al., 1996). These patterns have been hypothesized to have been the result of: (a) cohorts originating from different localities, which have different gene frequencies; (b) selection operating in the prerecruitment phase; and (c) recruitment representing very few families due to plankton from particular matings staying together during the dispersal phase.
Despite the lack of a passive planktonic larval stage, freshwater insects in south-east Queensland, Australia have shown very similar spatial patterns. For example, Schmidt et al. (1995) sampled populations of a mayfly species (Baetis sp.) from three streams from each of three subcatchments in each of two major drainages. The greatest genetic differentiation was on the smallest spatial scale, among streams within a subcatchment, and patchy recruitment was proposed as the most plausible mechanism. Further studies of populations of caddis fly larvae (Hughes et al., 1998) and water striders (Bunn & Hughes, 1997) showed a similar pattern. In these studies, where populations were also sampled among reaches within a single stream, the greatest differentiation was observed at the smallest spatial scale. The levels of differentiation observed at reach scales were an order of magnitude greater than those reported by Johnson & Black (1984a) and David et al. (1997) for marine species.
In addition to the unusual spatial patterns in genetic structure mentioned above, deviations from Hardy–Weinberg proportions were observed more frequently than expected by chance in all three freshwater insect species (Schmidt et al., 1995; Bunn & Hughes, 1997; Hughes et al., 1998). These deviations were not consistently seen at particular loci, nor at particular sites. Populations of the caddis fly Tasiagma ciliata (Tasimiidae) were also sampled from the same four reaches of one stream on two separate occasions so as to represent different cohorts (Hughes et al., 1998). The differences between sampling times for a given reach were at least as great as the differences among reaches for each time, both in terms of levels of genetic differentiation and deviations from Hardy–Weinberg proportions. These observations also support the interpretation that the individuals in any particular reach may be the offspring of a limited number of matings and therefore be unrepresentative of the total breeding adult population (Bunn & Hughes, 1997; Hughes et al., 1998).
The present study of the genetic structure of populations of the mayfly Bungona sp. (Ephemeroptera: Baetidae) was undertaken with the broad objective of testing further the patchy recruitment hypothesis. Populations were sampled at nested spatial scales and on three separate occasions. We predicted that the greatest genetic differentiation would be observed at the smallest spatial scale and that the spatial pattern of genetic structure within streams would differ between sampling times. Furthermore, we predicted that the patterns of differentiation among reaches would differ among loci.
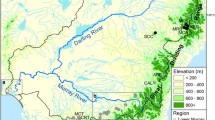
Earlier studies (Schmidt et al., 1995; Bunn & Hughes, 1997; Hughes et al., 1998) have all been conducted within an area of continuous dense forest within the Conondale Range. For all insect species studied, genetic differentiation across this region was negligible, indicating widespread adult dispersal between the two drainages on either side of the range. In the present study, we re-sampled streams from one subcatchment from the Conondale Range and three from the Blackall Range on the opposite side of the Mary River valley. These two low mountain ranges are separated by lowland areas of open woodland and cleared farmland (Fig. 1).
Methods
Study area
The Conondale Range forms part of the catchment divide separating the Brisbane River and Mary River in south-east Queensland, Australia. The Blackall Range joins the Conondale Range at its southern extreme and streams arising in it flow into the Mary River from the west (Fig. 1).
The climate of this region is described as subtropical, with hot wet summers and cool dry winters with a mean annual rainfall of approximately 1500 mm (Australian Bureau of Meteorology, 1983). The major vegetation types are a mixture of complex notophyll vine forest, wet sclerophyll forest, and dry sclerophyll forest (Czechura, 1991). Rainforest is widely distributed in upland areas (>500 m a.s.l.) and as riparian vegetation at lower altitudes.
The streamflow pattern is markedly seasonal, although there is considerable interannual variation (Pusey et al., 1993). During the wet season (summer/autumn), intense rain events (sometimes exceeding 400 mm/day) can cause major disturbances to the stream bed. For much of the year, however, the streams are reduced to a series of discrete but connected pools. Throughout this dry winter and spring period, the wetted area of the streams is usually only a small proportion of the active channel width. The Mary River valley between the Conondale and Blackall Ranges has been extensively cleared for agricultural purposes and existing forest fragments suggest there was a mixture of wet and dry sclerophyll forest type previous to settlement. The southern extreme of the two ranges has been extensively cleared for agriculture.
Sampling
Schmidt et al. (1995) referred to the common baetid mayfly in these streams as an unidentified species of Baetis. Subsequent taxonomic revisions of this family (Baetidae) in Australia (Suter, personal communication) now place this species in the genus Bungona. Electrophoretic data show at least another two species of baetids in these streams, but these are less common and easily distinguished from the study species. The family Baetidae is extremely widespread, although their biology is not well known (Campbell, 1988). Like all Ephemeroptera the adults have a short life-span, usually between one and three days and do not feed (Sweeney et al., 1986). The nymphs can be extremely abundant is some pools but abundance is very patchy (Bunn, unpublished data).
Samples of Bungona were collected on three occasions. In May 1993, we attempted to collect from three streams in each of four subcatchments in the Conondale Range (Hughes et al., 1995), as well as from three reaches in each stream. The spatial scales involved were as follows: Reach (within stream): 400–500 m apart in the same stream; Stream (within subcatchment): sites generally 1–5 km directly apart but up to 15 km via the channel network; Subcatchment (within drainage): 10–15 km directly apart but up to 50 km via the channel network; Drainage (Brisbane or Mary Rivers): headwater streams may only be a few hundred metres apart across the watershed but separated by >700 km, including over 200 km of ocean (see Fig. 1 and Hughes et al., 1995). This balanced nested design was not possible, because of the patchy distribution of the animals. We did, however, manage to collect from eight streams across the four subcatchments. In four of these (Stony, Booloumba, Bundaroo and Peters Creeks — see Fig. 1), samples were collected from three adjacent reaches. In Branch Creek West, Kilcoy Creek and Rum Crossing, samples were collected from two adjacent reaches.
In order to get a better estimate of within-stream variation, we re-sampled mayflies from four reaches in each of three streams (Stony, Bundaroo and Kilcoy Creeks — see Fig. 1) in February 1996. In October 1996, streams in both the Conondale Range (Booloumba and Peters Creeks) and the Blackall Range (Chinaman, Gherulla and Skene Creeks) were sampled. In most cases, four reaches were sampled in each stream.
Where possible, 100 individual larvae (each greater than 5 mm in length) were collected from each site and placed into snap lock plastic bags. These were immediately frozen in liquid nitrogen and returned to the laboratory where they were stored at −75°C until required for analysis.
Allozyme analysis
Animals were ground by hand in 30 μL of grinding buffer (2.44 g Trizma base, 0.37 g EDTA Free Acid, 5.36 g NH4Cl, 19.8 g glucose, 20 mL 0.022 M NaN3, in 1 litre of distilled water), and then spun at 10 000 r.p.m. for 15 min in a Sorvall refrigerated centrifuge at a temperature of 0–4°C. Allozyme electrophoresis was performed on Titan III cellulose acetate plates (Helena Laboratories). The enzymes run were glucose phosphate isomerase (PGI, IECC no. 5.3.1.9), phosphoglucomutase (PGM, no. 2.7.5.1), mannose phosphate isomerase (MPI, no. 5.3.1.8) peptidases B (substrate leucine-glycine-glycine) and C (substrate valine-leucine) (PEPB and C, IECC no. 3.4.11) and amylase (AMY, IECC no. 3.2.1.1). Detailed methods are given in Schmidt et al. (1995). All loci analysed were highly variable. Amy-1 and Mpi-1 had six alleles, PepB-1 and PepC-1 had seven alleles, Pgm-1 had nine alleles and Pgi-1 had 10 alleles. Raw data may be obtained from Jane Hughes on request.
Statistical analysis
BIOSYS (Swofford & Selander, 1989; release 1.7) was used to calculate FIS and FST values. Because of the different sampling designs used in the three years, analyses were slightly different in each case. For the May 1993 data, an hierarchical analysis was used to examine genetic differentiation at each of the four spatial scales: reach, stream, subcatchment and catchment. In order to look at variation within particular streams, we calculated Weir and Cockerham’s FST values, giving a jackknife estimate of the mean FST within each stream and a standard deviation. FST values for individual loci were tested for significance using the method of Waples (1987).
For the February 1996 data, when we were only interested in two spatial scales, we used the method of Rank (1992) and calculated FST values for each stream separately. The data from individual reaches within each stream were then pooled and a between-stream FST value was calculated. For the October 1996 data, which included the cross-valley comparisons, a similar method was employed, but with three spatial scales: reach, stream and mountain range. By using the above method we could examine variation among streams within each mountain range separately. In order to examine whether samples from a particular mountain range clustered together and were different from those from the adjacent range, the unweighted pair groups method (UPGMA) was used on a matrix of Nei’s genetic distances (based on the six polymorphic loci only). (Nei, 1978).
Results
Tests for Hardy–Weinberg proportions
On each of the three sampling occasions, significant deviations from Hardy–Weinberg proportions occurred more often than were expected by chance (Table 1). Both excesses and deficiencies of heterozygotes were observed, but deficiencies were more common (26 of 27 significant results in 1993, 25 of 26 in February 1996 and 28 of 28 in October 1996). Across all three years, no locus or site showed consistently significant results.
Differentiation among sites at different spatial scales
As predicted, the greatest genetic differentiation (largest FST value) was observed among reaches within streams sampled in May 1993 (Table 2). This was an order of magnitude greater than between streams within subcatchments or between subcatchments or drainages. Variation within individual streams at this time was examined separately. In all four streams, FST values were highly significant at two or three loci and each locus showed a highly significantly FST value in at least one stream.
As in the 1993 data, the patterns of genetic differentiation across loci in February 1996 were markedly different (Table 2). For the two streams where samples were obtained for both years, the results also differed markedly. In Bundaroo Creek, Pgi-1, Mpi-1 and PepB-1 showed significant variation in May 1993, whereas in February 1996, Mpi-1, PepB-1 and PepC- 1 were significant. In Stony Creek, only Mpi-1 and Amy-1 showed significant differentiation in May 1993 but Pgm-1 and Mpi-1 were significant in February 1996 (Table 2). Again the largest overall FST in February 1996 was among reaches within a single stream (Bundaroo Creek), but on this occasion the mean among-stream value was also quite high (0.025) and higher than the within-stream values for Kilcoy and Stony Creeks.
Overall, the FST values were lower in October 1996 than on any other sampling occasion (Table 3). However, in three of the five streams sampled, at least one locus showed highly significant differentiation within the stream. Mean differentiation among the streams in the Blackall Range was an order of magnitude higher than between the two streams in the Conondale Range. This difference was largely the effect of variation at the PepB-1 locus, which did not vary significantly within any of the five streams (Table 3).
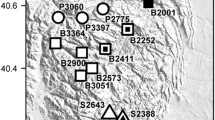
The highest FST value in October 1996 was between the Blackall Range and the Conondale Range and this was also reflected in the UPGMA (Fig. 2). As may be expected if the lowland areas represent some barrier to adult movement, all the samples in the Blackall Range were more similar to one another than they were to any of the samples from the Conondale Range.
Discussion
Fine-scale genetic structure and patchy recruitment
As was predicted from previous studies, there was a large number of significant deviations from Hardy–Weinberg proportions at all three sampling times. The fact that most of these were positive indicates an overall deficiency of heterozygotes, which would result if a small number of families with differing allele frequencies were mixed. A similar result was recorded for the same species in an earlier study (Schmidt et al., 1995). Once again, there was no tendency for a particular locus to exhibit deviations, as may be expected if there were problems with gel interpretation or if there were null alleles present (Richardson et al., 1986). Similarly, no particular site stood out as showing deficiencies across all loci, as would occur if we were dealing with two or more cryptic species. Furthermore, when a site was examined on more than one occasion, the same loci did not usually show heterozygote deficiencies. Together, these observations support the hypothesis that the larvae in a particular reach are the offspring of only a few matings: where the families have different alleles at a locus, there will be less heterozygotes than if all possible matings were represented.
Similar results have been observed in populations of a species of caddis fly larvae and a species of water strider from streams in the same area (Bunn & Hughes, 1997; Hughes et al., 1998). It was suggested that, in these subtropical streams, only a few matings can occur at the reach scale at any time because emergence of adults is not a synchronous event. This contrasts with the situation in many temperate streams where synchronous emergence of adults occurs over a very short time period and oviposition events are likely to represent offspring of the entire breeding population. In such streams, patterns of high numbers of deviations from Hardy–Weinberg proportions and significant within-stream differentiation would not be expected. Only one similar study has been carried out on insects in temperate streams, on the stonefly Yoraperla brevis (Peltoperlidae), which inhabits montane streams in the Rocky Mountains, USA (Hughes et al., 1999). In contrast with the subtropical species, from analysis of five loci from 27 samples of Yoraperla (i.e. 135 comparisons) only five showed significant deviations from Hardy–Weinberg proportions. This number is less than would be expected by chance.
The observed fine-scale patterns in genetic structure in these studies could also occur even if large numbers of adults were present, if successful recruitment was limited by the number of suitable oviposition sites. This has been suggested in species of Baetis (and presumably other stream Baetidae) where adults require oviposition sites on large stones in flowing water that are partially exposed (Peckarsky et al., in press). Elevated high flows during the breeding period may greatly reduce the number of oviposition sites and thus the number of successful matings represented in the subsequent larval population. This explanation is unlikely, however, as all Australian Baetidae observed to date (including species of Bungona) broadly disperse their eggs rather than lay discrete eggs masses (P. Suter, personal communication).
As also predicted by the patchy recruitment hypothesis, FST values were highly variable among loci. For example, FST values within Booloumba Creek in 1993 varied from 0 (at Pgi-1 and Pgm-1) to 0.282 at PepB-1 and 0.188 at Mpi-1. The loci showing significant FST values also differed between times for the streams that were sampled more than once. The data from 1993 supported earlier suggestions that the greatest level of differentiation may be at the smallest spatial scale (Schmidt et al., 1995). However, in the earlier study, the smallest spatial scale examined was the stream, whereas in the present study the smallest scale was the reach. The February 1996 data also supported this suggestion to some extent, as the largest differentiation was within Bundaroo Creek. However, the other two streams showed lower levels of differentiation than that between streams.
The results from the October 1996 sampling did not follow the pattern of high levels of differentiation among reaches within a stream. In fact the mean FST values for all five streams were less than 0.01 and in only three of the five streams were any significant FST values observed. In addition, for both the two Conondale streams and the three Blackall streams, the between-stream FST values were larger than the within-stream values. This result differs from the earlier two sampling times and from the two sampling times where the caddis fly Tasiagma was examined on the same spatial scale (Hughes et al., 1998).
One possible explanation is that the October 1996 sampling was carried out after a large recruitment event and streams may have had more families represented in each reach (i.e. the result of high numbers of adults). If this was the case, however, the relative number of deviations from Hardy–Weinberg proportions would also be expected to be lower. Although the percentage for October 1996 (25%) was lower than that for February 1996 (36%), this was not different from that observed in 1993 (24%). A second explanation is that on this occasion the genotypes of the matings just happened not to differ by chance. Even if only a few matings are represented in each reach, it is possible that they may not differ among reaches. This would be more likely at loci that are not highly variable. In fact, overall, the three loci with the lowest frequency of the most common allele (i.e. the most variable) (Pgm-1, Mpi-1, PepB-1) also have the highest number of significant FST values. This explanation does not necessarily predict a lower number of deviations from Hardy–Weinberg proportions and so may be more likely than an increased number of families. It could only be really tested with one or more additional sampling exercises. A third possibility is that only a few matings are represented in each reach but more larval movement has occurred among reaches. Stream flows in the months prior to the October 1996 sampling were much higher than the long-term (1959–98) monthly means, whereas they were only between 2% and 20% of the means in the 6 months prior to May 1993 and February 1996 (Queensland Department of Natural Resources, unpublished streamflow data). Movement during times of elevated flows may have been sufficient to homogenize gene frequencies but not to bring populations in reaches to Hardy–Weinberg proportions. Within each reach we would see a Wahlund Effect, due to mixing of families with different allele frequencies.
There was evidence of greater genetic differences between populations of Bungona sp. from different blocks of continuous forest than between populations within them. Overall, the FST values were small and indicated significant adult dispersal both within and between the two mountain ranges. Although the results of the UPGMA suggest that samples within the Blackall Range were more similar to each other than to samples from the Conondale Range and vice versa, the low FST values at all loci but PepB-1 suggest that limited dispersal may not be the explanation. Selection on PepB-1 cannot be ruled out. The FST values among streams in the Blackall Range were close to an order of magnitude greater than those among the two streams in the Conondale Range. The two Conondale Range streams were closer to one another than were the Blackall Range streams and are part of the same subcatchment, with continuous rainforest between them. The Blackall Range streams all flow independently into the Mary River and are separated by areas of more open, wet sclerophyll forest. Thus the higher differentiation among Blackall Range streams may reflect terrestrial habitat discontinuities between them.
Studies of movement of adult stream insects indicate a general trend for upstream dispersal (Müller, 1992), and limited movement away from the stream channel and riparian zone (Jackson & Resh, 1989) (Cameron, 1985; Collier & Smith, 1996). Recent work in rainforest streams in south-east Queensland suggests that adult stream insects do not venture far from the stream and most travel, above the channel or in adjacent riparian vegetation (Smith, 1995). The limited evidence presented here of restricted dispersal of Bungona sp. across the open valley separating the two mountain ranges may simply reflect the tendency of adults to fly upstream to oviposit in higher altitude streams. However, ability of adults to move across the lowland reaches is also likely to be constrained by the absence of suitable, continuous riparian habitat.
References
AUSTRALIAN BUREAU OF METEOROLOGY (1983). The climate of Brisbane, Queensland (Capital City Series). Australian Government Publishing Service, Canberra.
Bunn, S. E. and Hughes, J. M. (1997). Dispersal and recruitment in streams: evidence from genetic studies. J North Am Benthol Soc, 16, 338–346.
Campbell, I. (1988). Ephemeroptera. In: Walton, D.W. (ed) Zoological Catalogue of Australia, vol. 6, Australian Government Publishing Service.
Cameron, L. (1985). Habitat usage and foraging behaviour of three fantails (Rhipidura: Pachycephalidae). In: Keast, A., Recher, H. F., Ford, H. and Saunders, D. (eds) Birds of Eucalypt Forests and Woodlands: Ecology, Conservation Management. pp. 177–191. Surrey Beatty & Sons Pty Ltd, Chippery Norton, NSW.
Collier, K. J. and Smith, B. J. (1996). Riparian vegetation use by adult Ephenosoptera, Plenoptera and Trichoptera alongside some central north Island streams. NIWA Science and Technology Series no. 34. New Zealand.
Czechura, G. V. (1991). The Blackall–Conondale Ranges: frogs, reptiles and fauna conservation, pp. 311–324. In: Warren, G. and Kershaw, P. (eds) The Rainforest Legacy vol. 2 – Flora and Fauna of the Rainforest Australian Government Publishing Service, Canberra.
David, P., Perdieu, M. A., Pernot, A. F. and Jarne, P. (1997). Spatial and temporal population genetic structure in the marine bivalve Spisula ovalis. Evolution, 51, 1318–1322.
Doherty, P., Planes, S. and Mather, P. B. (1996). Gene flow and larval duration in seven species of fish from the Great Barrier Reef. Ecology, 76, 2373–2391.
Gibbs, H. L., Gibbs, K. E., Siebenmann, M. and Collins, L. (1998). Genetic differentiation among populations of the rare mayfly Siphlonisca aerodromia Needham. J N Am Benthol Soc, 17, 464–474.
Hughes, J. M., Bunn, S. E., Kingston, D. M. and Hurwood, D. A. (1995). Genetic differentiation and dispersal among populations of Paratya australiensis (Atyidae) in rainforest streams in south-east Queensland, Australia. J N Am Benthol Soc, 14, 158–173.
Hughes, J. M., Bunn, S. E., Hurwood, D. A., Choy, S. and Pearson, R. G. (1996). Genetic differentiation among populations of Caridina zebra (Decapoda: Atyidae) in tropical rainforest streams, northern Australia. Freshwater Biol, 36, 289–296.
Hughes, J. M., Bunn, S. E., Hurwood, D. A. and Cleary, C. (1998). Dispersal and recruitment of Tasiagma ciliata (Trichoptera: Tasimiidae) in rainforest streams, south east Queensland, Australia. Freshwater Biol, 39, 117–127.
Hughes, J. M., Mather, P. B., Sheldon, A. L. and Allendorf, F. W. (1999). Genetic structure of stonefly (Yoraperla brevis) populations: the extent of gene flow among adjacent montane streams. Freshwater Biol, 41, 1–10.
Jackson, J. K. and Resh, V. H. (1989). Distribution and abundance of adult aquatic insects in the forest adjacent to a northern California stream. Environ Entomol, 18, 278–283.
Johnson, M. S. and Black, R. (1984). Pattern beneath chaos: the effect of recruitment on genetic patchiness in an intertidal limpet. Evolution, 38, 1371–1383.
Müller, K. (1992). The colonization cycle of freshwater insects. Oecologia, 52, 202–207.
Nei, M. (1978). Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics, 89, 583–590.
Peckarsky, B. L., Taylor, B. W., Caudill, C. C. in press. Hydrologic and behavioural constraints on oviposition of stream insects: implications for dispersal. Oecologia. in press.
Pusey, B., Arthington, A. H. and Read, M. G. (1993). Spatial and temporal variation in fish assemblage structure in the Mary River, south-eastern Queensland: the influence of habitat structure. Environ Biol Fishes, 37, 355–380.
Rank, N. (1992). A hierarchical analysis of genetic variation in the montane leaf beetle Chrysomela aeneicollis (Coleoptera: Chrysomelidae). Evolution, 46, 1097–1111.
Richardson, B. J., Baverstock, P. R. and Adams, M. (1986). Allozyme Electrophoresis. A Handbook for Animal Systematics and Population Studies. Academic Press, Sydney.
Schmidt, S. K., Hughes, J. M. and Bunn, S. E. (1995). Gene flow among conspecific populations of Baetis sp. (Ephemeroptera): adult flight and larval drift. J North Am Benthol Soc, 14, 147–157.
Smith, I. L. (1995). The Distribution and Movement Pattern of Adult Aquatic Insects and Their Effect on Terrestrial Insectivore Food Webs Among Riparian Habitats Adjacent to Two Mountain Streams in South-East Queensland, Australia. Honours Thesis, Faculty of Environmental Sciences, Griffith University.
Sweeney, B. W., Frank, D. H. and Vannote, R. L. (1986). Population genetic structure of two mayflies (Ephemerella subvaria, Emylophella verisimilis) in the Delaware River drainage basin. J North Am Benthol Soc, 4: 253–262.
Swofford, D. L. and Selander, R. B. (1989). BIOSYS-1. A computer program for the analysis of allelic variation in population genetics and biochemical systematics Release 1.7. University of Illinois, Urbana, IL.
Waples, R. S. (1987). A multispecies approach to the analysis of gene flow in marine shore fishes. Evolution, 41, 385–400.
Acknowledgements
This work was funded by an Australian Research Council grant to JMH and SEB. Permits to collect were kindly provided by the Queensland National Parks and Wildlife Service and the Queensland Department of Natural Resources. Digital maps of the study area and detailed flow data were also provided by the Queensland Department of Natural Resources; special thanks are given to John Amprimo and Peter Fiedler. We also thank Dr Phil Suter, La Trobe University, for unpublished information on oviposition behaviour of Australian Baetidae, Chris Marshall for help in the field and Jill Shephard for producing the map of sampling sites.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hughes, J., Bunn, S., Cleary, C. et al. A hierarchical analysis of the genetic structure of an aquatic insect Bungona (Baetidae: Ephemeroptera). Heredity 85, 561–570 (2000). https://doi.org/10.1046/j.1365-2540.2000.00782.x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1046/j.1365-2540.2000.00782.x
- Springer Nature Switzerland AG