Summary
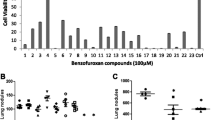
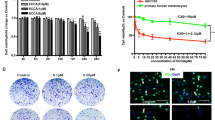
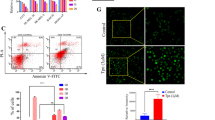
Cutaneous melanoma, the most aggressive form of skin cancer, is characterized by activating BRAF mutations. Despite the initial success of selective BRAF inhibitors, only few patients exhibited complete responses, whereas many showed disease progression. Melanoma is one of the few types of cancer in which p53 is not frequently mutated, but p53 inactivation can be indirectly achieved by a stable activation of MDM2 induced by a deletion in CDKN2A (Cyclin Dependent Kinase Inhibitor 2A) locus, encoding for p16INK4A and p14ARF, two tumor suppressor genes. In this study, we tested the efficacy of the previously synthesized tetra-substituted pyrrole derivatives, 8 g, 8 h and 8i, in melanoma cell lines, and we compared the effects of the most active of these, the 8i compound, with that exerted by Nutlin 3, a well-known inhibitor of p53-MDM2 interaction. The obtained results showed that 8i potentiates the inhibitory effect of Nutlin 3 and the combined use of 8i and Nutlin 3 triggers apoptosis and significantly impairs melanoma viability. Finally, the 8i compound reduces p53-MDM2 interaction and induces p53-HSP90 complex formation, suggesting that the observed raise in p53 transcriptional activity could be mediated by HSP90. Because the main feature of melanoma is the resistance to most chemotherapeutics, our studies suggest that the 8i tetra-substituted pyrrole derivative, restoring p53 functions and its transcriptional activities, may have potential application, at least as adjuvant, in the treatment of human melanoma.









Similar content being viewed by others
References
Haluska FG, Tsao H, Wu H, Haluska FS, Lazar A, Goel V (2006) Genetic alterations in signaling pathways in melanoma. Clin Cancer Res 12(7 Pt 2):2301s–2307s. https://doi.org/10.1158/1078-0432.CCR-14-1215
Ascierto PA, Kirkwood JM, Grob JJ, Simeone E, Grimaldi AM, Maio M, Palmieri G, Testori A, Marincola FM, Mozzillo N (2012) The role of BRAF V600 mutation in melanoma. J Transl Med 10:85. https://doi.org/10.1186/1479-5876-10-85
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O'Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur G, BRIM-3 Study Group. (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364(26):2507–2516. https://doi.org/10.1056/NEJMoa1103782
Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M (2012) Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380(9839):358–365. https://doi.org/10.1016/S0140-6736(12)60868-X
Fedorenko IV, Paraiso KH, Smalley KS (2011) Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol 82(3):201–209. https://doi.org/10.1016/j.bcp.2011.05.015
Poulikakos PI, Solit DB (2011) Resistance to MEK inhibitors: should we co-target upstream? Sci Signal 4(166):pe16. https://doi.org/10.1126/scisignal.2001948
Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, DiCara D, Ramos AH, Lawrence MS, Cibulskis K, Sivachenko A, Voet D, Saksena G, Stransky N, Onofrio RC, Winckler W, Ardlie K, Wagle N, Wargo J, Chong K, Morton DL, Stemke-Hale K, Chen G, Noble M, Meyerson M, Ladbury JE, Davies MA, Gershenwald JE, Wagner SN, Hoon DSB, Schadendorf D, Lander ES, Gabriel SB, Getz G, Garraway LA, Chin L (2012) A landscape of driver mutations in melanoma. Cell 150(2):251–263. https://doi.org/10.1016/j.cell.2012.06.024
Gembarska A, Luciani F, Fedele C, Russell EA, Dewaele M, Villar S, Zwolinska A, Haupt S, de Lange J, Yip D, Goydos J, Haigh JJ, Haupt Y, Larue L, Jochemsen A, Shi H, Moriceau G, Lo RS, Ghanem G, Shackleton M, Bernal F, Marine JC (2012) MDM4 is a key therapeutic target in cutaneous melanoma. Nat Med 18(8):1239–1247. https://doi.org/10.1038/nm.2863
Murray-Zmijewski F, Slee EA, Lu X (2008) A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol 9(9):702–712. https://doi.org/10.1038/nrm2451
Fischer M (2017) Census and evaluation of p53 target genes. Oncogene 36(28):3943–3956. https://doi.org/10.1038/onc.2016.502
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA (2004) In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303(5659):844–848. https://doi.org/10.1126/science.1092472
Ji Z, Njauw CN, Taylor M, Neel V, Flaherty KT, Tsao H (2012) p53 rescue through HDM2 antagonism suppresses melanoma growth and potentiates MEK inhibition. J Invest Dermatol 132(2):356–364. https://doi.org/10.1038/jid.2011.313
Lu M, Breyssens H, Salter V, Zhong S, Hu Y, Baer C, Ratnayaka I, Sullivan A, Brown NR, Endicott J, Knapp S, Kessler BM, Middleton MR, Siebold C, Jones EY, Sviderskaya EV, Cebon J, John T, Caballero OL, Goding CR, Lu X (2013) Restoring p53 function in human melanoma cells by inhibiting MDM2 and cyclin B1/CDK1-phosphorylated nuclear iASPP. Cancer Cell 23(5):618–633. https://doi.org/10.1016/j.ccr.2013.03.013
Persico M, Ramunno A, Maglio V, Franceschelli S, Esposito C, Carotenuto A, Brancaccio D, De Pasquale V, Pavone LM, Varra M, Orteca N, Novellino E, Fattorusso C (2013) New anticancer agents mimicking protein recognition motifs. J Med Chem 56(17):6666–6680. https://doi.org/10.1021/jm400947b
Hind CK, Carter MJ, Harris CL, Chan HT, James S, Cragg MS (2015) Role of the pro-survival molecule Bfl-1 in melanoma. Int J Biochem Cell Biol 59:94–102. https://doi.org/10.1016/j.biocel.2014.11.015
Esposito T, Sansone F, Franceschelli S, Del Gaudio P, Picerno P, Aquino RP, Mencherini T (2017) Hazelnut (Corylus avellana L.) shells extract: phenolic composition, antioxidant effect and cytotoxic activity on human Cancer cell lines. Int J Mol Sci 18(2). pii: E392. https://doi.org/10.3390/ijms18020392
Piccinelli AL, Pagano I, Esposito T, Mencherini T, Porta A, Petrone AM, Gazzerro P, Picerno P, Sansone F, Rastrelli L, Aquino RP (2016) HRMS profile of a hazelnut skin Proanthocyanidin-rich fraction with antioxidant and anti-Candida albicans activities. J Agric Food Chem 64(3):585–595. https://doi.org/10.1021/acs.jafc.5b05404
Proto MC, Gazzerro P, Di Croce L, Santoro A, Malfitano AM, Pisanti S, Laezza C, Bifulco M (2012) Interaction of endocannabinoid system and steroid hormones in the control of colon cancer cell growth. J Cell Physiol 227(1):250–258. https://doi.org/10.1002/jcp.22727
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzym Regul 22:27–55
Gazzerro P, Malfitano AM, Proto MC, Santoro A, Pisanti S, Caruso MG, Notarnicola M, Messa C, Laezza C, Misso G, Caraglia M, Bifulco M (2010) Synergistic inhibition of human colon cancer cell growth by the cannabinoid CB1 receptor antagonist rimonabant and oxaliplatin. Oncol Rep 23(1):171–175
Fiore D, Ramesh P, Proto MC, Piscopo C, Franceschelli S, Anzelmo S, Medema JP, Bifulco M, Gazzerro P (2018) Rimonabant kills Colon Cancer stem cells without inducing toxicity in Normal Colon organoids. Front Pharmacol 8:949. https://doi.org/10.3389/fphar.2017.00949
Proto MC, Fiore D, Piscopo C, Franceschelli S, Bizzarro V, Laezza C, Lauro G, Feoli A, Tosco A, Bifulco G, Sbardella G, Bifulco M, Gazzerro P (2017) Inhibition of Wnt/β-catenin pathway and histone acetyltransferase activity by Rimonabant: a therapeutic target for colon cancer. Sci Rep 7(1):11678. https://doi.org/10.1038/s41598-017-11688-x
Dal Piaz F, Cotugno R, Lepore L, Vassallo A, Malafronte N, Lauro G, Bifulco G, Belisario MA, De Tommasi N (2013) Chemical proteomics reveals HSP70 1A as a target for the anticancer diterpene oridonin in Jurkat cells. J Proteome 82:14–26. https://doi.org/10.1016/j.jprot.2013.01.030
Dal Piaz F, Vera Saltos MB, Franceschelli S, Forte G, Marzocco S, Tuccinardi T, Poli G, Nejad Ebrahimi S, Hamburger M, De Tommasi N, Braca A (2016) Drug affinity responsive target stability (DARTS) identifies Laurifolioside as a new Clathrin heavy chain modulator. J Nat Prod 79(10):2681–2692. https://doi.org/10.1021/acs.jnatprod.6b00627
Shangary S, Wang S (2009) Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol 49:223–241. https://doi.org/10.1146/annurev.pharmtox.48.113006.094723
van Leeuwen IM, Higgins M, Campbell J, Brown CJ, McCarthy AR, Pirrie L, Westwood NJ, Laín S (2011) Mechanism-specific signatures for small-molecule p53 activators. Cell Cycle 10:1590–1598
de Lange J, Ly LV, Lodder K, Verlaan-de Vries M, Teunisse AF, Jager MJ, Jochemsen AG (2012) Synergistic growth inhibition based on small-molecule p53 activation as treatment for intraocular melanoma. Oncogene 31(9):1105–1116. https://doi.org/10.1038/onc.2011.309
Pai MY, Lomenick B, Hwang H, Schiestel R, McBride W, Loo JA, Huang J (2015) Drug affinity responsive target stability (DARTS) for small-molecule target identification. Methods Mol Biol 1263:287–298. https://doi.org/10.1007/978-1-493-2269-7_22
Arthur CR, Gupton JT, Kellogg GE, Yeudall WA, Cabot MC, Newsham IF, Gewirtz DA (2007) Autophagic cell death, polyploidy and senescence induced in breast tumor cells by the substituted pyrrole JG-03-14, a novel microtubule poison. Biochem Pharmacol 74(7):981–991
Yoshii SR and Mizushima N ( 2017) Monitoring and measuring autophagy. Int J Mol Sci 18(9). Pii: E1865. https://doi.org/10.3390/ijms18091865
Manzano JL, Layos L, Bugés C, de Los Llanos Gil M, Vila L, Martínez-Balibrea E, Martínez-Cardús A (2016) Resistant mechanisms to BRAF inhibitors in melanoma. Ann Transl Med 4(12):237. https://doi.org/10.21037/atm.2016.06.07
Griffin M, Scotto D, Josephs DH, Mele S, Crescioli S, Bax HJ, Pellizzari G, Wynne MD, Nakamura M, Hoffmann RM, Ilieva KM, Cheung A, Spicer JF, Papa S, Lacy KE, Karagiannis SN (2017) BRAF inhibitors: resistance and the promise of combination treatments for melanoma. Oncotarget 8(44):78174–78192. https://doi.org/10.18632/oncotarget.19836
Hocker T, Tsao H (2007) Ultraviolet radiation and melanoma: a systemic review and analysis of reported sequence variants. Hum Mutat 28:588
Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP (2009) Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer 9(12):862–873. https://doi.org/10.1038/nrc2763
Lu M, Miller P, Lu X (2014) Restoring the tumour suppressive function of p53 as a parallel strategy in melanoma therapy. FEBS Lett 588(16):2616–2621. https://doi.org/10.1016/j.febslet.2014.05.008
Hata AN, Rowley S, Archibald HL, Gomez-Caraballo M, Siddiqui FM, Ji F, Jung J, Light M, Lee JS, Debussche L, Sidhu S, Sadreyev RI, Watters J, Engelman JA (2017) Synergistic activity and heterogeneous acquired resistance of combined MDM2 and MEK inhibition in KRAS mutant cancers. Oncogene 36(47):6581–6591. https://doi.org/10.1038/onc.2017.258
Smalley KS, Contractor R, Haass NK, Kulp AN, Atilla-Gokcumen GE, Williams DS, Bregman H, Flaherty KT, Soengas MS, Meggers E, Herlyn M (2007) An organometallic protein kinase inhibitor pharmacologically activates p53 and induces apoptosis in human melanoma cells. Cancer Res 67(1):209–217. https://doi.org/10.1158/0008-5472.CAN-06-1538
Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, Myklebost O, Heimbrook DC, Vassilev LT (2006) Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci U S A 103(6):1888–1893. https://doi.org/10.1073/pnas.0507493103
Ji Z, Kumar R, Taylor M, Rajadurai A, Marzuka-Alcalá A, Chen YE, Njauw CN, Flaherty K, Jönsson G, Tsao H (2013) Vemurafenib synergizes with nutlin-3 to deplete survivin and suppresses melanoma viability and tumor growth. Clin Cancer Res 19(16):4383–4391. https://doi.org/10.1158/1078-0432.CCR-13-0074
Sutton SK, Carter DR, Kim P, Tan O, Arndt GM, Zhang XD, Baell J, Noll BD, Wang S, Kumar N, McArthur GA, Cheung BB, Marshall GM (2016) A novel compound which sensitizes BRAF wild-type melanoma cells to vemurafenib in a TRIM16-dependent manner. Oncotarget 7(32):52166–52178. https://doi.org/10.18632/oncotarget.10700
Agaësse G, Barbollat-Boutrand L, El Kharbili M, Berthier-Vergnes O, Masse I (2017) p53 targets TSPAN8 to prevent invasion in melanoma cells. Oncogenesis. 6(4):e309. https://doi.org/10.1038/oncsis.2017.11
Spencer SL, Cappell SD, Tsai FC, Overton KW, Wang CL, Meyer T (2013) The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell 155(2):369–383. https://doi.org/10.1016/j.cell.2013.08.062
Whitesell L, Lindquist SL (2005) HSP90 and the chaperoning of cancer. Nat Rev Cancer 5(10):761–772. https://doi.org/10.1038/nrc1716
Schopf FH, Biebl MM, Buchner J (2017) The HSP90 chaperone machinery. Nat Rev Mol Cell Biol 18(6):345–360. https://doi.org/10.1038/nrm.2017.20
Blagosklonny MV, Toretsky J, Bohen S, Neckers L (1996) Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc Natl Acad Sci U S A 93(16):8379–8383
Hagn F, Lagleder S, Retzlaff M, Rohrberg J, Demmer O, Richter K, Buchner J, Kessler H (2011) Structural analysis of the interaction between Hsp90 and the tumor suppressor protein p53. Nat Struct Mol Biol 18(10):1086–1093. https://doi.org/10.1038/nsmb.2114
Müller L, Schaupp A, Walerych D, Wegele H, Buchner J (2004) Hsp90 regulates the activity of wild type p53 under physiological and elevated temperatures. J Biol Chem 279(47):48846–48854. https://doi.org/10.1074/jbc.M407687200
Walerych D, Kudla G, Gutkowska M, Wawrzynow B, Muller L, King FW, Helwak A, Boros J, Zylicz A, Zylicz M (2004) Hsp90 chaperones wild-type p53 tumor suppressor protein. J Biol Chem 279(47):48836–48845. https://doi.org/10.1074/jbc.M407601200
Acquaviva J, Smith DL, Jimenez JP, Zhang C, Sequeira M, He S, Sang J, Bates RC, Proia DA (2014) Overcoming acquired BRAF inhibitor resistance in melanoma via targeted inhibition of Hsp90 with ganetespib. Mol Cancer Ther 13(2):353–363. https://doi.org/10.1158/1535-7163.MCT-13-0481
Mbofung RM, McKenzie JA, Malu S, Zhang M, Peng W, Liu C et al (2017) HSP90 inhibition enhances cancer immunotherapy by upregulating interferon response genes. Nat Commun 8(1):451. https://doi.org/10.1038/s41467-017-00449-z
Tseng HY, Jiang CC, Croft A, Tay KH, Thorne RF, Yang F, Liu H, Hersey P, Zhang XD (2010) Contrasting effects of nutlin-3 on TRAIL- and docetaxel-induced apoptosis due to upregulation of TRAIL-R2 and Mcl-1 in human melanoma cells. Mol Cancer Ther 9(12):3363–3374. https://doi.org/10.1158/1535-7163.MCT-10-0646
Tokalov SV, Abolmaali ND (2010) Protection of p53 wild type cells from taxol by nutlin-3 in the combined lung cancer treatment. BMC Cancer 10:57
Huang B, Deo D, Xia M, Vassilev LT (2009) Pharmacologic p53 activation blocks cell cycle progression but fails to induce senescence in epithelial cancer cells. Mol Cancer Res 7:1497–1509
Boisvert-Adamo K, Longmate W, Abel EV, Aplin AE (2009) Mcl-1 is required for melanoma cell resistance to anoikis. Mol Cancer Res 7(4):549–556. https://doi.org/10.1158/1541-7786.MCR-08-0358
Borthakur G, Duvvuri S, Ruvolo V, Tripathi DN, Piya S, Burks J, Jacamo R, Kojima K, Ruvolo P, Fueyo-Margareto J, Konopleva M, Andreeff M (2015) MDM2 inhibitor, Nutlin 3a, induces p53 dependent autophagy in acute leukemia by AMP kinase activation. PLoS One 10(10):e0139254. https://doi.org/10.1371/journal.pone.0139254
Davaadelger B, Perez RE, Zhou Y, Duan L, Gitelis S, Maki CG (2017) The IGF-1R/AKT pathway has opposing effects on Nutlin-3a-induced apoptosis. Cancer Biol Ther 18(11):895–903. https://doi.org/10.1080/15384047.2017.1345397
Vincent KM, Postovit LM (2017) Investigating the utility of human melanoma cell lines as tumour models. Oncotarget 8(6):10498–10509. https://doi.org/10.18632/oncotarget.14443
Acknowledgments
MCP was supported by a research fellowship from Regione Campania (POR Campania FSE 2007–2013). The authors thank Prof Bruno Maresca (University of Salerno) for his critical review and dr. Chiara Piscopo (University of Salerno) for her technical support.
Funding
The work was supported by the Department of Pharmacy, University of Salerno, Italy.
Author information
Authors and Affiliations
Contributions
MCP designed and conducted research, analyzed and interpreted data, wrote the paper. DF, GF, PC, performed research and analyzed data. AR, CF and MP supervised the project and gave substantial contribution to analysis and interpretation of data. PG and SF designed and supervised the project in its entirety, provided financial support, wrote and critically reviewed the paper. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Maria Chiara Proto declares that she has no conflict of interest. Donatella Fiore declares that she has no conflict of interest. Giovanni Forte declares that he has no conflict of interest. Paola Cuozzo declares that she has no conflict of interest. Anna Ramunno declares that she has no conflict of interest. Caterina Fattorusso declares that she has no conflict of interest. Patrizia Gazzerro declares that she has no conflict of interest. Maria Pascale declares that she has no conflict of interest. Silvia Franceschelli declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 322 kb)
Rights and permissions
About this article
Cite this article
Proto, M.C., Fiore, D., Forte, G. et al. Tetra-substituted pyrrole derivatives act as potent activators of p53 in melanoma cells. Invest New Drugs 38, 634–649 (2020). https://doi.org/10.1007/s10637-019-00813-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00813-4