Abstract
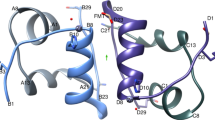
X-ray analysis of the pancreatic hormone glucagon shows that in crystals the polypeptide adopts a mainly helical conformation, which is stabilised by hydrophobic interactions between molecules related by threefold symmetry. A model is presented in which the glucagon molecule exists in dilute solutions as an equilibrium population of conformers with little retention of structure, and in which the helical conformation is stabilised by hydrophobic interactions either as an oligomer or as a complex with the receptor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bromer, W. W., Sinn, L. G., and Behrens, O. K., J. Am. chem. Soc., 79, 2807–2810 (1957).
Pohl, S. L., Birnbaumer, L., and Rodbell, M., Science, 164, 566–569 (1969).
Gratzer, W. B., Beavan, G. H., Rattle, H. W. E., and Bradbury, E. M., Eur. J. Biochem, 3, 276–283 (1968).
Blanchard, M. H., and King, M. V., Biochem. biophys. Res. Commun., 25, 298–303 (1966).
Panijpan, B., and Gratzer, W. B., Eur. J. Biochem., 45, 547–553 (1974).
Gratzer, W. B., and Beavan, G. H., J. biol. Chem., 244, 6675–6679 (1969).
Bornet, H., and Edelhoch, H., J. biol. Chem., 246, 1785–1792 (1971).
Schneider, A. B., and Edelhoch, H., J. biol. Chem., 247, 4986–4991 (1972).
King, M. V., J. molec. Biol., 1, 375–377 (1959); 11, 549–561 (1965).
Haugen, W. P., and Lipscomb, W. N., Acta Crystallogr., A 25 (Suppl.) S185 (1969).
Blundell, T. L., Hodgkin, D. C., Dodson, G. G., and Mercola, D. A., Adv. Protein Chem., 26, 279–402 (1972).
Staub, A., Sinn, L., and Behrens, O. K., J. biol. Chem., 214, 619–632 (1955).
Tickle, I. J., Acta Crystallogr., B 31, 329–331 (1975).
North, A. C. T., Phillips, D. C., and Mathews, F. S., Acta Crystallogr., A 24, 351–359 (1968).
Harding, M., thesis, Oxford Univ. (1962).
Singh, A. K., and Ramasehan, S., Acta Crystallogr., 21, 279–280 (1966).
Matthews, B. W., Acta Crystallogr., 20, 230–239 (1966).
North, A. C. T., Acta Crystallogr., 18, 212–216 (1965).
Blow, D. M., and Crick, F. H. C., Acta Crystallogr., 12, 794–802 (1959).
Richards, F. M., J. molec. Biol., 37, 225–230 (1968).
Swann, J. C., and Hammes, G. G., Biochemistry, 8, 1–7 (1969).
Frank, B. H., and Pekar, A. H., J. biol. Chem., 249, 4846–4850 (1974).
Rodbell, M., Birnbaumer, L., Pohl, S. L., and Sundby, F., Proc. natn. Acad. Sci. U.S.A., 68, 909–913 (1971).
Birnbaumer, L., Pohl, S. L., and Rodbell, M., J. biol. Chem., 244, 3468–3475 (1968).
Epand, R. M., Epand, R. F., and Vijay Iaxmi, G., Archs Biochem. Biophys., 154, 132–136 (1973).
Epand, R. M., and Wheeler, G. E., Biochim. biophys. Acta, 393, 236–240 (1975).
Contaxis, C. C., and Epand, R. M., Can. J. Biochem., 52, 456–468 (1974).
Schwyzer, R., Pure appl. Chem., 6, 265–295 (1963).
Birgen, A. S. V., Roberts, G. C. K., and Feeney, J., Nature, 253, 754–755 (1975).
Levey, G. S., Fletcher, M. A., Klein, I., Ruiz, E., and Schenk, A., J. biol. Chem., 249, 2665–2673 (1974).
Beddell, C. R., et al., J. med. Chem., 18, 417–423 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sasaki, K., Dockerill, S., Adamiak, D. et al. X-ray analysis of glucagon and its relationship to receptor binding. Nature 257, 751–757 (1975). https://doi.org/10.1038/257751a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/257751a0
This article is cited by
-
Density functional theory calculations of spectral, NLO, reactivity, NBO properties and docking study of Vincosamide-N-Oxide active against lung cancer cell lines H1299
SN Applied Sciences (2020)
-
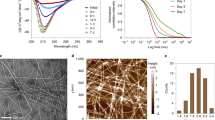
The peptide hormone glucagon forms amyloid fibrils with two coexisting β-strand conformations
Nature Structural & Molecular Biology (2019)
-
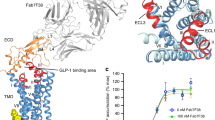
Current understanding of the structure and function of family B GPCRs to design novel drugs
Hormones (2018)
-
Structure and mechanism for recognition of peptide hormones by Class B G-protein-coupled receptors
Acta Pharmacologica Sinica (2012)
-
Structures of human insulin-degrading enzyme reveal a new substrate recognition mechanism
Nature (2006)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.