Abstract
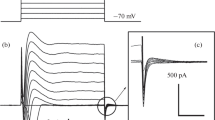
We performed experiments to elucidate the calcium influx pathways in freshly dispersed rabbit corneal epithelial cells. Three possible pathways were considered: voltage-gated Ca++ channels, Na+/Ca++ exchange, and nonvoltage-dependent Ca++-permeable channels. Whole cell inward currents carrying either Ca++ or Ba++ were not detected using voltage clamp techniques. We also used imaging technology and the Ca++-sensitive ratiometric dye fura 2 to measure changes in intracellular Ca++ concentration ([Ca]i). Bath perfusion with NaCl Ringer's solution containing the calcium channel agonist Bay-K-8644 (1 μm), or Ni++ (40 μm), a blocker of many voltage-dependent calcium channels, did not affect [Ca++]i. Membrane depolarization with a KCl Ringer's bath solution resulted in a decrease in [Ca++]i. These results are inconsistent with the presence of voltage gated Ca++ channels. Nonvoltage gated Ca++ entry, on the other hand, would be reduced by membrane depolarization and enhanced by membrane hyperpolarization. Agents which hyperpolarize via stimulation of K+ current, such as flufenamic acid, resulted in an increase in ratio intensity. The cells were found to be permeable to Mn++ and bath perfusion with 5 mm Ni++ decreased [Ca++]i suggesting that the Ca++ conductance was blocked. These results are most consistent with a nonvoltage gated Ca++ influx pathway. Finally, replacing extracellular Na+ with Li+ resulted in an increase in [Ca++]i if the cells were first Na+-loaded using the Na+ ionophore monensin and ouabain, a Na+-K+-ATPase inhibitor. These results suggest that Na+/Ca++ exchange may also regulate [Ca++] in this cell type.
Similar content being viewed by others
References
Ambudkar, I.S., Baum, B J. 1988. ATP-dependent calcium transport in rat parotid basolateral membrane vesicles is modulated by membrane potential. J. Membrane Biol. 102:59–69
Artalejo, C.R., Adams, M.E., Fox, A.P. 1994. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature 367:72–76
Clapham, D.E. 1993. A mysterious new influx factor? Nature 364:763–764
Farrugia, G., Rae, J.L. 1992. Regulation of a potassium-selective current in rabbit comeal epithelium by cyclic GMP, carbachol, and diltiazem. J. Membrane Biol. 129:99–107
Farrugia, G., Rae, J.L. 1993. Effect of volume changes on a potassium current in rabbit corneal epithelial cells. Am. J. Physiol. 264:C1238-C1245, 1993
Fasolato, C, Innocenti, B., Pozzan, T. 1994. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends in Physiol. Sci. 15:77–83
Grynkiewicz, G., Poenie, M., Tsien, R. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440–3450
Hoth, M., Penner, R. 1992. Depletion of intracellular calcium stores activates a calcium current in rat mast cells. Nature 355:353–355
Luckhoff, A., Clapham, D.E. 1992. Inositol 1,3,4,5-tetrakisphosphate activates an endothelial Ca2+-permeable channel. Nature 355:356–358
Marks, P.W., Maxfield, F.R. 1991. Preparation of solutions with free calcium concentration in the nanomolar range using 1,2-Bis-(oaminophenoxy)ethane-N,N,N′,N′-tetraacetic acid. Analytical Biochemistry 193:61–71
Neher, E. 1992. Controls on calcium influx. Nature 355:298–299
Nilius, B. 1990. Permeation properties of a non-selective cation channel in human vascular endothelial cells. Pfluegers Arch. 416:609–611
Rae, J.L., Cooper, K., Gates, P., Watsky, M. 1991. Low access resistance perforated patch recordings using amphotericin B. J. Neurosci. Methods 37:15–25
Rae, J.L., Dewey, J., Cooper, K., Gates, P. 1990a. A non-selective cation channel in rabbit comeal endothelium activated by internal calcium and inhibited by internal ATP. Exp. Eye Res. 50:373–384
Rae, J.L., Dewey, J., Rae, J.S., Nesler, M., Cooper, K. 1990b. Single potassium channels in corneal epithelium. Invest Opthalmol. Vis. Sci. 31:1799–1809
Rae, J.L., Farrugia, G. 1992. Whole-cell potassium current in rabbit corneal epithelium activated by fenamates. J. Membrane Biol. 129:81–97
Reinach, P.S., Holmberg, N., Chiesa, R. 1991. Identification of a calmodulin-sensitive Ca2+-transporting ATPase in the plasma membrane of bovine corneal epithelial cell. Biochemica et Biophysica Acta 1068:1–8
Reinach, P.S., Socci, R.R., Keith, C., Scanlon, M. 1992. Adrenergic receptor-mediated increase of intracellular Ca2+ concentration in isolated bovine corneal epithelial cells. Comp. Biochem. Physiol. 102A:709–714
Sage, S.O., van Breeman, C., Cannell, M.B. 1991. Sodium-calcium exchange in cultured bovine pulmonary artery endothelial cells. J. Physiol. 440:569–580
Schulze, D., Kofuji, P., Hadley, R., Kirby, M.S., Kieval, R.S., Doering, A., Niggli, E., Lederer, W.J. 1993. Sodium/calcium exchanger in heart muscle: molecular biology, cellular function, and its special role in excitation-contraction coupling. Cardiovascular Research 27:1726–1734
Author information
Authors and Affiliations
Additional information
The authors are grateful to Chris Bartling for expert technical assistance with the imaging experiments, Helen Hendrickson for cell preparation, and Jonathon Monck for helpful discussions regarding imaging technology. This work was supported by National Institutes of Health grants EYO3282, EYO6005, DK08677, and an unrestricted award from Research to Prevent Blindness.
Rights and permissions
About this article
Cite this article
Rich, A., Rae, J.L. Calcium entry in rabbit corneal epithelial cells: Evidence for a nonvoltage dependent pathway. J. Membarin Biol. 144, 177–184 (1995). https://doi.org/10.1007/BF00232803
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00232803