Abstract
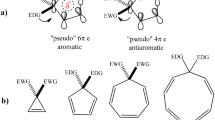
Nucleus Independent Chemical Shift (NICS) values, pioneered by Schleyer, provide detailed insights into electronic structures of transition states. These show that the [2+2+2]-cycloaddition transition states, studied early by Schleyer and by us, have aromatic transition structures and that fused cyclopropanes are aromatic in the transition structure, while fused four-membered rings are antiaromatic. The nucleophilic ring-opening of 1- and 2-cyanobicyclo[1.1.0]butanes, studied earlier by Hoz, and ring openings of cyanocyclopropane, cyanocyclobutane, and 2-cyanobicyclo[2.2.0]hexane by hydroxide were investigated at the B3LYP/6-31+G* level. Orbital interactions through bonds explain relative facilities of ring opening.
Similar content being viewed by others
Author information
Authors and Affiliations
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Sawicka, D., Houk, K. Aromaticity and Antiaromaticity in Small Ring Transition States, Assessed by NICS Values and Energetics. J Mol Model 6, 158–165 (2000). https://doi.org/10.1007/s0089400060158
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s0089400060158