Abstract
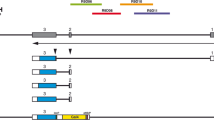
To assess the functional domains of the proteins encoded by E(spl) and HLH-m5, two genes of the Enhancer of split complex [E(SPL)-C] of Drosophila melanogaster, a number of variants have been made by in vitro mutagenesis, transformed into the germ line of the wild-type, and genetically combined with a chromosomal deletion lacking four of the genes of the E(SPL)-C. All constructs used attenuated the neurogenic phenotype associated with this deletion. However, constructs encoding proteins with truncated carboxy-termini exibited in all cases a higher activity than constructs encoding the full length version of the protein. Neutralization of the basic domain severely reduced, but did not completely abolish the rescuing activity of E(spl), while proteins in which a proline residue within the basic domain had been changed to either threonine or asparagine were slightly less efficient in their rescuing activity than the corresponding wild-type versions. We discuss the possible significance of these results for the function of the protein domains.
Similar content being viewed by others
References
Alonso MC, Cabrera CV (1988) The achaete-scute gene complex of Drosophila melanogaster comprises four homologous genes. EMBO J 7:2585–2591
Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H (1990) The protein Id: a negative regulator of of helix-loophelix DNA binding proteins. Cell 61:49–59
Bengal E, Ransone L, Scharfmann R, Dwarki VJ, Tapscott SJ, Weintraub H, Verma IM (1992) Functional antagonism between c-Jun and MyoD proteins: a direct physical association. Cell 68:507–519
Cagan RL, Ready DF (1989) Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev 3:1099–1112
Cai M, Davis RW (1990) Yeast centromere binding protein CBF1, of the helix-loop-helix protein family, is required for chromosome stability and methionine prototrophy. Cell 61:437–446
Campos-Ortega JA, Knust E (1990a) Genetics of early neurogenesis in Drosophila melanogaster. Ann Rev Genet 24:387–407
Campos-Ortega JA, Knust E (1990b) Defective ommatidial cell assembly leads to defective morphogenesis: a phenotypic analysis of the E(spl) Dmutation of Drosophila melanogaster. Roux's Arch Dev Biol 198:286–294
Candy M, Vässin H, Brand M, Tuma R, Jan LY, Jan YN (1988) daughterless, a gene essential for both neurogenesis and sex determination in Drosophila, has sequence similarities to myc and the achaete-scute complex. Cell 55:1061–1067
Celis J de, Mari-Beffa M, Garcia-Bellido A (1991) Cell autonomous role of Notch, an epidermal growth factor homolog, in sensory organ differentiation in Drosophila. Proc Natl Acad Sci USA 88:632–636
Corbin V, Michelson AM, Abmayr SM, Neel B, Alcamo E, Maniatis T, Young MW (1991) A role for the Drosophila neurogenic genes in mesoderm differentiation. Cell 67:311–323
Delidakis C, Artavanis-Tsakonas S (1992) The Enhancer of split [E(spl)] locus of Drosophila encodes seven independent helixloop-helix proteins. Proc Natl Acad Sci 89:8731–8735
Dietrich U, Campos-Ortega JA (1984) The expression of neurogenic loci in imaginal epidermal cells of Drosophila melanogaster. J Neurogenet 1:315–332
Doren M van, Ellis HM, Posakony JW (1991) The Drosophila extramacrochaetae protein antagonizes sequence-specific DNA binding by daughterless/achaete-scute protein complexes. Development 113:245–255
Ellis HM, Spann DR, Posakony JW (1990) extramacrochaetae, a negative regulator of sensory organ development in Drosphila, defines a new class of helix-loop-helix proteins. Cell 61:27–38
Garrell J, Campuzano S (1991) The helix-loop-helix domain: a common motif for bristles, muscles and sex. BioEssays 13:493–498
Garrell J, Modolell J (1990) The Drosophila extramacrochaetae locus, an antagonist of proneural genes that, like these genes, encodes a helix-loop-helix protein. Cell 61:39–48
Gonzalez F, Romani S, Cubas P, Modolell J, Campuzano S (1989) Molecular analysis of asense, a member of the achaete-scute complex of Drosophila melanogaster, and its novel role in optic lobe development. EMBO J 8:3553–3562
Hartenstein V, Posakony JW (1990) A dual function of the Notch gene in Drosophila sensillum development. Dev Biol 142:13–30
Hartenstein AY, Rugendorff A, Tepass U, Hartenstein V (1992) The function of the neurogenic genes during epithelial development in the Drosophila embryo. Development 116:1203–1220
Heitzler P, Simpson P (1991) The choice of cell fate in the epidermis of Drosophila. Cell 64:1083–1092
Klämbt C, Knust E, Tietze K, Campos-Ortega JA (1989) Closely related transcripts encoded by the neurogenic gene complex Enhancer of split of Drosophila melanogaster. EMBO J 7:203–210
Klemenz R, Weber U, Gehring WJ (1987) The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acid Res 15:3947–3959
Knust E, Bremer KA, Vässin H, Ziemer A, Tepaß U, Campos-Ortega JA (1987a) The Enhancer of split locus and neurogenesis in Drosophila melanogaster. Dev Biol 122:262–273
Knust E, Tietze K, Campos-Ortega JA (1987b) Molecular analysis of the neurogenic locus Enhancer of split of Drosophila melanogaster. EMBO J 6:4113–4123
Knust E, Schrons H, Grawe F, Campos-Ortega JA (1992) Seven genes of the Enhancer of split complex of Drosophila melanogaster encode helix-loop-helix proteins. Genetics 132:505–518
Laski FA, Rio DC, Rubin GM (1986) Tissue specificity of Drosophila P element transposition is regulated at the level of mRNA splicing. Cell 44:7–19
Lindsley DL, Zimm GG (1992) The genome of Drosophila. Academic Press, San Diego
Ludwig SR, Wessler SR (1990) Maize R gene family: Tissue specific helix-loop-helix proteins. Cell 62:849–851
Lüscher B, Eisenmann RN (1990) New light on Myc and Myb. Part I. Myc. Genes Dev 4:2025–2035
Meer van der (1977) Optical clean and permanent wholemount preparation for phase contrast microscopy of cuticular structures of insect larvae. Drosophila Inf Serv 52:160
Mellor J, Jiang W, Funk M, Rathjen J, Barnes CA, Hinz T, Hegemann JH, Philippsen P (1990) CPF1, a yeast protein which functions in centromeres and promoters. EMBO J 9:4017–4026
Murre C, McCaw PS, Baltimore D (1989) A new DNA-binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell 56:777–783
Olson EN (1990) MyoD family: a paradigm for development? Genes Dev 4:1454–1461
Preiss A, Hartley DA, Artavanis-Tsakonas S (1988) The molecular genetics of Enhancer of split, a gene required for embryonic neural development in Drosophila. EMBO J 7:3917–3928
Ruohola H, Bremer KA, Baker D, Swedlow JR, Jan LY, Jan YN (1991) Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell 66:433–449
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain termination inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Sayers JR, Schmidt W, Eckstein F (1988) 5′-3′ exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acid Res 16:791–802
Schrons H, Knust E, Campos-Ortega JA (1992) The Enhancer of split complex and adjacent genes in the 96F region of Drosophila melanogaster are required for segregation of neural and epidermal progenitor cells. Genetics 132:481–503
Shepard SB, Broverman SA, Muskavitch MAT (1989) A tripartite interaction among alleles of Notch, Delta and Enhancer of split during imaginal development of Drosophila melanogaster. Genetics 122:429–438
Spradling AC (1986) P element-mediated transformation. In: Roberts DB (ed) Drosophila. A practical approach. IRL Press, Oxford, pp 175–197
Tietze K, Oellers N, Knust E (1992) Enhancer of split D, a dominant mutation of Drosophila, and its use in the study of functional domains of a helix-loop-helix protein. Proc Natl Acad Sci USA 89:6152–6156
Villares R, Cabrera CV (1987) The achaete-scute gene complex of Drosophila melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell 50:415–424
Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S, Zhuang Y, Lassar A (1991) The MyoD gene family: nodal points during specification of the muscle cell lineage. Science 251:761–766
Welshons WJ (1956) Dosage experiments with split mutants in the presence of an enhancer of split. Drosophila Inf Serv 30:157–158
Welshons WJ (1965) Analysis of a gene in Drosophila. Science 150:1122–1129
Xu T, Caron LA, Fehon RG, Artavanis-Tsakonas S (1992) The involvement of the Notch locus in Drosophila oogenesis. Development 115:993–922
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tietze, K., Schrons, H., Campos-Ortega, J.A. et al. A functional analysis of the genes Enhancer of split and HLH-m5 during early neurogenesis in Drosophila melanogaster . Roux's Arch Dev Biol 203, 10–17 (1993). https://doi.org/10.1007/BF00539885
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00539885