Abstract
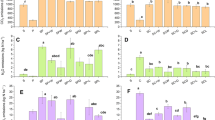
Subsoil acidity is a serious constraint to crop production, and is difficult to correct by conventional liming practices. Thus, a different approach to ameliorating acid subsoils was evaluated. Subsoil material of an acid Ultisol (pH 4.4) was packed into 50-cm long columns, then leached with solutions of CaCl2, CaCO3 (suspension) or Ca fulvates prepared from chicken manure, cowpea green manure, or sewage sludge. The total water applied was 30.26 cm (or 800 ml) in 2 days. Thereafter, the columns were dismantled and cut into 5-cm segments for chemical analysis. The results indicated that only 2% of the added Ca from CaCO3 moved past the 15-cm depth, compared to 68% from CaCl2 and 35–75% from Ca fulvates. Correspondingly, CaCO3 precipitated all KCl-extractable Al in the top 5 cm, but had no effect beyond the 10-cm depth. The CaCl2 displaced a small but significant portion of extractable Al from the top 15 cm and redeposited some of that Al in lower depths. Similar to CaCO3, Ca fulvates from chicken manure and green mamure only decreased extractable Al significantly in the top 10-cm layers, but had little effect beyond that depth. By contrast, the Ca fulvate from sewage sludge decreased Al down to the 45-cm depth. In terms of reducing Al saturation as a percentage of total extractable cations (effective cation exchange capacity), the Ca fulvates were as effective as CaCO3 in the 0-to 5-cm layer, and more effective than CaCl2 in any soil layer because of the increased exchangeable Ca and/or decreased Al. In general, surface application of common organic material-derived Ca fulvates can increase subsoil Ca and decrease the Al saturation percentage. However, Mg depletion and enrichment of unwanted metals (e.g., Na or heavy metals) may be a problem when leaching with these organic sources.
Similar content being viewed by others
References
Adams F (1984)Crop response to lime in the Southern United States In: Adams F (ed) Soil acidity and liming. Soil Sci Soc Am, Madison, Wis, pp 211–265
Alva AK, Summer ME, Miller WP (1990) Reactions of gypsum or phosphogypsum in highly weathered acid subsoils. Soil Sci Soc Am J 54:993–998
Bohn HL, McNeal BL, O'Connor GA (1985) Soil chemistry,2nd edn. Wiley and Sons, New York, pp 243–260
Cahn MD, Boulding DR, Cravo MS (1993) Amelioration of subsoil acidity in an Oxisol of the humid tropics. Biol Fertil Soils 15:153–159
Farina MPW, Channon P (1988) Acid-subsoil amelioration: II. Gypsum effects on growth and subsoil chemical properties. Soil Sci Soc Am J 52:175–180
Foy CD (1984) Physiological effects of hydrogen, aluminum, and manganese toxicities in acid soils.In: Adams F (ed) Soil acidity and liming. Soil Sci Soc Am, Madison, Wis, pp 57–97
Hue NV, (1992) Correcting soil acidity of a highly weathered Ultisol with ckicken manure and sewage sludge. Commun Soil Sci Plant Anal 23:241–264
Hue NV, Amien I (1989) Aluminum detoxification with green manures. Commun Soil Sci Plant Anal 20:1499–1511
Hue NV, Adams F, Evans CE (1985) Sulfate retention by an acid BE horizon of an Ultisol. Soil Sci Soc Am J 49:1196–1200
Hue NV, Craddock GR, Adams F (1986) Effect of organic acids on aluminum toxicity in subsoils. Soil Sci Soc Am J 50:28–34
Kamprath EJ (1984) Crop response to lime in soils in the tropics. In: Adams F (ed) Soil acidity and liming. Soil Sci Soc Am, Madison, Wis, pp 349–368
Kinraide TB, Parker DR (1987) Nonphytotoxicity of the aluminum sulfate ion, AlSO +4 . Physiol Plant 71:207–212
Mathews BW, Joost RE (1990) The effects of leaching surface-applied amendments on subsoil aluminum and alfalfa growth in a Louisiana Ultisol. Commun Soil Sci Plant Anal21:567–581
Motekaitis RJ, Martell AE(1984) Complexes of aluminum (III) with hydroxy carboxylic acids. Inorg Chem 23:18–23
Parfitt RL, Smart RS (1978) The mechanism of sulfate adsorption on Fe oxides. Soil Sci Soc Am J 42:48–50
Pavan MA, Bingham FT, Pratt PF (1982) Toxicity of aluminum to coffee in Ultisols and Oxisols amended with CaCO3, MgCO3, and CaSO4·2H2O. Soil Sci Soc Am J 46:1201–1207
Pavan MA, Bingham FT, Pratt PF (1984) Redistribution of exchangeable calcium, magnesium, and aluminum following lime or gypsum applications to a Brazilian Oxisol. Soil Sci Soc Am J 48:33–38
Rajan SSS (1978) Sulfate adsorbed on hydrous alumina, ligands displaced, and changes in surface charge. Soil Sci Soc Am J 42:39–44
Sanchez PA, Logan TJ (1992) Myths and science about the chemistry and fertility of soils in the tropics. In: Lal R, Sanchez PA (eds) Myths and science of soils of the tropics. Soil Sci Soc Am Spec Publ No. 29, Madison, Wis, pp 35–46
Smith CJ, Goh KM, Bond WJ, Freney JR, Tuomi SS (1993) Amelioration of acidity in subsurface soil with organic and inorganic calcium compounds. In: Program and Abstract, 3rd Int symp plant-soil interactions at low pH. 12–16 Sept 1993, Brisbane, Australia. Australian Soc Soil Sci,p 129
Tan KH, Edwards JH, Bennett OL (1985) Effect of sewage sludge on mobilization of surface-applied calcium in a Greenville soil. Soil Sci 139:262–269
van der Watt HVH, Barnard RO, Cronje IJ Dekker J, Croft GJB, van der Walt MM (1991) Amelioration of subsoil acidity by application of a coal-derived calcium fulvate to the soil surface. Nature (London) 350:146–148
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, J., Hue, N.V. Ameliorating subsoil acidity by surface application of calcium fulvates derived from common organic materials. Biol Fert Soils 21, 264–270 (1996). https://doi.org/10.1007/BF00334902
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00334902